The yin and yang in the life of proteins
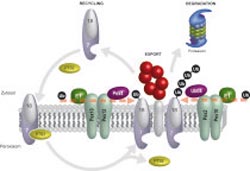
Peroxisomal protein transport model: The import receptor for peroxisomal PTS2 proteins is the two-part receptor complex Pex18/Pex7. After the PTS2 protein has been released into the interior of the peroxisome, the receptor is exported. Docking an ubiquitin (Ub) with Pex18 sends out the signal that the receptor can be recycled for new import reactions. The docking of the individual ubiquitins is determined by the E1 enzyme, the E2 enzyme Pex4p as well as the E3 enzymes Pex12/Pex10. If an ubiquitin chain forms at Pex18, the proteasome breaks down Pex18. Docking of the ubiquitin chain is effected through the E1 enzyme, the E2 enzyme Ubc4 as well as the E3 enzymes Pex2/Pex10.<br><br>© RUB, Grafik: Platta<br>
Recycling or “scrap press”: physicians at the Ruhr-Universität have found out which molecular mechanisms decide about the fate of the import receptor Pex18. Pex18 is responsible for the import of proteins into specific cell components, namely peroxisomes. Two opposing regulatory circuits determine whether the receptor remains active or is broken down after the transport has been completed.
“Thus, the picture of the regulation of the protein import into peroxisomes has been completed and integrated to form one single model,” says Junior Professor Dr Harald Platta from the RUB Faculty of Medicine. Together with Prof Dr Ralf Erdmann and other colleagues he reports in the journal “Traffic”.
Ubiquitin signals determine the fate of the receptors
Because they don’t have their own DNA, peroxisomes have to import all proteins that are necessary for them to fulfil their function. For this purpose, the cell is equipped with dynamic import receptors such as Pex18. They bind proteins in the cytoplasm and transport them to the peroxisome. The RUB team had demonstrated in a previous study that the signal protein ubiquitin subsequently decides about the future fate of the receptors: if a single ubiquitin protein docks with the receptor, the receptor gets recycled; it migrates back into the cytoplasm and launches a new transport process. If an ubiquitin chain docks with the receptor, a signal is sent out for the receptor to be broken down by the proteasome, an “intracellular scrap press”, so to speak. Prior to this discovery, it had not been understood in what way the cell determines on the molecular level what happens to the receptor.
Recycling or “scrap press”: It all depends on the enzyme cascade
The RUB physicians found out that different enzyme cascades catalyse the two ubiquitin modifications of Pex18. In both cases, it is a three-step process: the E1 enzyme activates the ubiquitin signal which is subsequently transferred by the E2 enzyme and, eventually, coordinated by the E3 enzyme to dock with the receptor. By analysing yeast cells, the Bochum physicians found out that E2 and E3 enzymes occur in different variations, whereas there is only one type of the E1 enzyme. The docking of one single ubiquitin and an ubiquitin chain is determined by different combinations of E2 and E3 enzymes. “That means two opposing molecular machines determine the fate of the import receptor Pex18,” says Harald Platta. “This discovery illustrates just how precisely the receptor’s control is calibrated and how precisely the regulation associated with it is effected for the entire peroxisomal function. This project constitutes a crucial foundation for further research into the molecular causes of peroxisomal disorders.”
Peroxisomes: The cell’s multi-functional tools
Peroxisomes are important reaction states within the cell. They may contain up to 50 enzymes which are crucial for breaking down of fatty acids, for the disposal of hydrogen peroxide and the generation of plasmalogens which are an important component of the brain’s white matter. A disruption of the protein import in peroxisomes has a negative impact on the entire metabolism and may be fatal – especially for newborns.
Bibliographic record
F. El Magraoui, R. Brinkmeier, A. Schrötter, W. Girzalsky, T. Müller, K. Marcus, H.E. Meyer, R. Erdmann, H.W. Platta (2013): Distinct ubiquitination cascades act on the PTS2-co-receptor Pex18p, Traffic, DOI: 10.1111/tra.12120
Media Contact
More Information:
http://www.ruhr-uni-bochum.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















