Biophysics: Testing how well biomarkers work
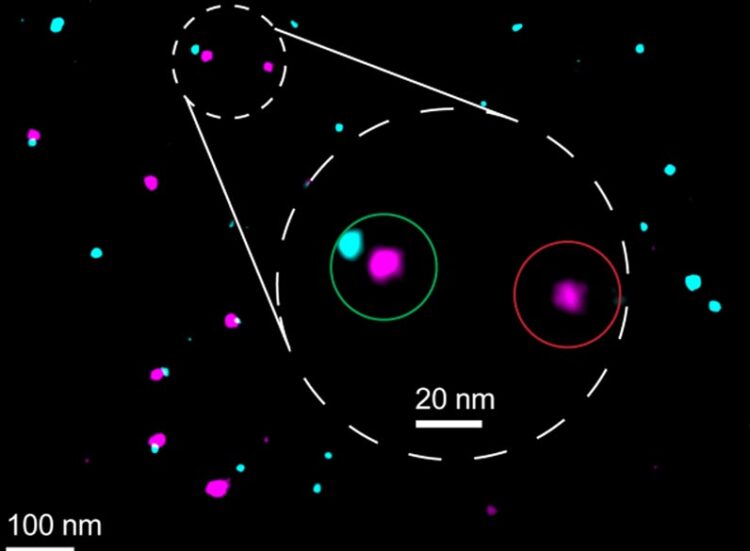
Under the microscope, the reference and marker appear as differently colored points of light. | © Joschka Hellmeier & Sebastian Strauss (LMU/MPI)
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy.
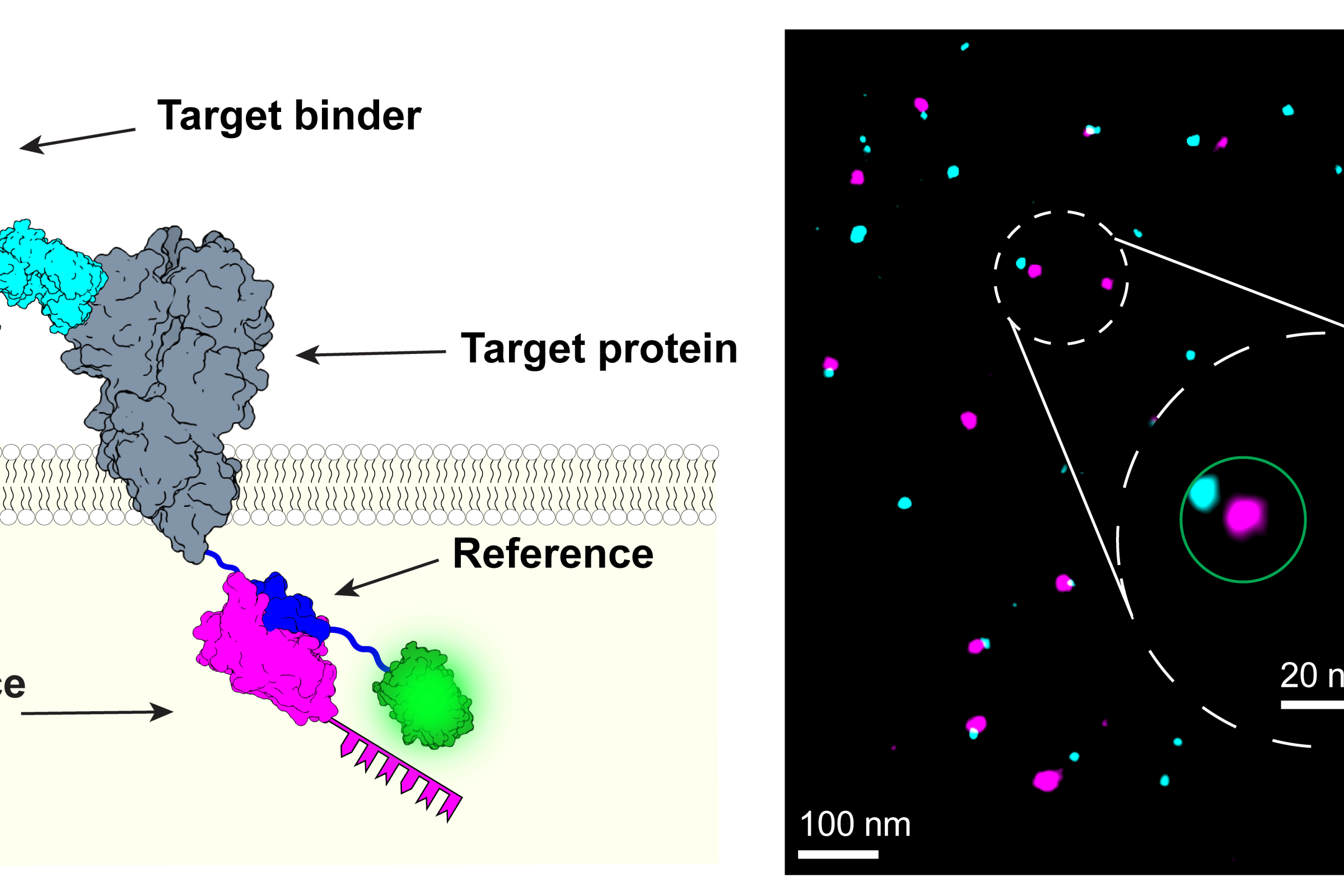
Modern microscopy techniques make it possible to examine the inner workings of cells in astonishing detail. “We can now observe the arrangement and interaction of individual proteins under the microscope,” says Professor Ralf Jungmann, Chair of Molecular Physics of Life at LMU and Max Planck Fellow at the MPI of Biochemistry. The biophysicist’s team recently developed the revolutionary RESI (Resolution Enhancement by Sequential Imaging) method. This technique can be used to improve the resolution of fluorescence microscopy down to the Ångström scale – far below the classical diffraction limit of light. DNA-conjugated marker molecules, which the researchers attach precisely to the molecules they want to understand better, are crucial for this.
Under the microscope, the reference and marker appear as differently colored points of light. © Joschka Hellmeier & Sebastian Strauss (LMU/MPI)
Jungmann’s team has now presented a technique in the journal Nature Methods that can be used to quantify how well biomarker molecules bind to the target proteins. “This is absolutely crucial if you want to make quantitatively reliable statements,” explains the physicist. If you know the labeling efficiency, you can carry out spatially resolved proteomics in this way. This allows you to find out not only what individual proteins do in a cell, but also to what extent they are present and how their quantity and behavior change under certain circumstances. “But this is only possible if we can assess how well the labeling has worked.” This is because only labeled proteins emit flashes of light under the microscope and thus become visible.
Reliable and versatile
The method developed by Jungmann’s team makes this assessment possible by adding a reference biomarker to the target proteins. This marker “glows” in a different color during microscopy, so that successfully marked proteins appear in two colors. Jungmann’s team demonstrated this using the membrane protein CD86, among others: The reference produces a pink fluorescence, the actual marker a bluish one. This creates a pattern of innumerable pink and blue points of light. Where the marking did not work, only the reference lights up individually. The marking efficiency is calculated from the ratio of double and single illuminated molecules.
The technique can be applied to a variety of different target molecules, biomarkers, and samples and is compatible with a whole range of super-resolution microscopy methods. Ralf Jungmann
The authors of the study are certain that the new quantification method has paved the way for significantly expanding the potential of their super-resolution microscope method: “Now we can also consider specific biomedical applications in which the quantitative detection of proteins and processes is of great importance,” says Jungmann. This includes cancer research, for example, where information about interactions between proteins on the cell surface and drugs with molecular resolution is essential for the development of new types of medication.
Joschka Hellmeier, Sebastian Strauss, Shuhan Xu, Luciano A. Masullo, Eduard M. Unterauer, Rafal Kowalewski & Ralf Jungmann: Quantification of absolute labeling efficiency at the single-protein level. Nature Methods, 2024.
More on this subject:
- Beyond the limits of light : LMU physicists are using a new technique to enhance fluorescence microscopy to the Ångstrom scale – far below the resolution limit of conventional light microscopy.
- New synapse type discovered by spatial proteomics : Researchers led by Ralf Jungmann have developed a super-resolution imaging method (SUM-PAINT) to map protein distributions in neurons and discovered a new type of synapse.
- Microscopy: Overcoming the traditional resolution limit for the fast co-tracking of molecules : Researchers at LMU have developed an innovative method to simultaneously track rapid dynamic processes of multiple molecules at the molecular scale.
https://www.lmu.de/en/newsroom/news-overview/news/biophysics-testing-how-well-biomarkers-work.html
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Sea slugs inspire highly stretchable biomedical sensor
USC Viterbi School of Engineering researcher Hangbo Zhao presents findings on highly stretchable and customizable microneedles for application in fields including neuroscience, tissue engineering, and wearable bioelectronics. The revolution in…

Twisting and binding matter waves with photons in a cavity
Precisely measuring the energy states of individual atoms has been a historical challenge for physicists due to atomic recoil. When an atom interacts with a photon, the atom “recoils” in…

Nanotubes, nanoparticles, and antibodies detect tiny amounts of fentanyl
New sensor is six orders of magnitude more sensitive than the next best thing. A research team at Pitt led by Alexander Star, a chemistry professor in the Kenneth P. Dietrich…





















