After 70 years, advanced carbon-based magnetic material finally synthesized
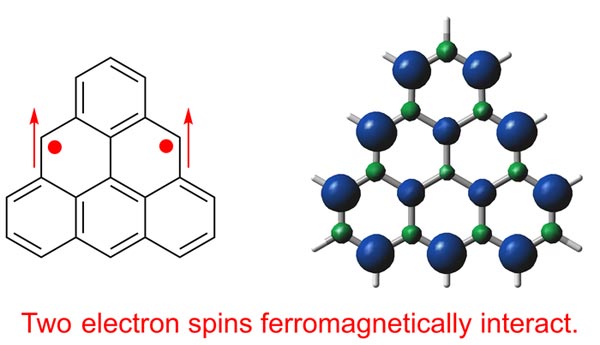
Structure and spin density distribution of triangulene
Credit: Shinobu Arikawa et al.
Researchers from Osaka University and Osaka City University synthesize and crystallize a molecule that is otherwise too unstable to fully study in the laboratory, and is a model of a revolutionary class of magnets.
Since the first reported production in 2004, researchers have been hard at work using graphene and similar carbon-based materials to revolutionize electronics, sports, and many other disciplines. Now, researchers from Japan have made a discovery that will advance the long-elusive field of nanographene magnets.
In a study recently published in Journal of the American Chemical Society, researchers from Osaka University and collaborating partners have synthesized a crystalline nanographene with magnetic properties that have been predicted theoretically since the 1950s, but until now have been unconfirmed experimentally except at extremely low temperatures.
Graphene is a single layer, two-dimensional sheet of carbon rings arranged in a honeycomb lattice. Why does graphene excite researchers? Graphene has impressive properties—it exhibits efficient, long-distance charge transport and has a much higher strength than similarly thick steel. Nanostructures of graphene have edges that exhibit magnetic and electronic properties that researchers would like to exploit. However, graphene nanosheets are difficult to prepare and it’s difficult to study their zigzag edge properties. Overcoming these challenges by using a simpler, yet advanced, model system known as triangulene is something the researchers at Osaka University aimed to address.
“Triangulene has long eluded synthesis in a crystalline form because of its uncontrolled polymerization,” say both Shinobu Arikawa and Akihiro Shimizu, two key authors of the study. “We prevented this polymerization by steric protection—bulking up the molecule—and did so in a way that didn’t affect its underlying properties.”
The researchers’ triangulene derivative is stable at room temperature but must be kept in an inert atmosphere because it slowly degrades when exposed to oxygen. Nevertheless, crystallization was possible—which enabled confirmation of its theoretically predicted properties, such as localization of unpaired electrons on the zigzag edges of the molecule.
“By measuring its optical and magnetic properties, we confirmed that our molecule is in the triplet ground state,” explains Ryo Shintani, senior author. “This is an electronic state that can serve as an experimentally tractable model for zigzag-edged nanographene.”
These results have important applications. Researchers can extend the long-sought synthetic procedure reported here to increase the number of carbon rings in the molecule and perform chemical syntheses of advanced forms of nanographene. In so doing, Osaka University and Osaka City University researchers may be able to synthesize materials that are foundational for future advanced electronics and magnets, and supplement the silicon that’s ubiquitous in modern electronics.
The article, “Synthesis and isolation of a kinetically stabilized crystalline triangulene,” was published in Journal of the American Chemical Society at DOI: https://doi.org/10.1021/jacs.1c10151
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan’s leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan’s most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en
Journal: Journal of the American Chemical Society
DOI: 10.1021/jacs.1c10151
Method of Research: Experimental study
Subject of Research: Not applicable
Article Title: Synthesis and isolation of a kinetically stabilized crystalline triangulene
Article Publication Date: 12-Nov-2021
Media Contact
Saori Obayashi
Osaka University
gi-strategy@cgin.osaka-u.ac.jp
Office: 81-661-055-886
Original Source
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















