Cancer risk takes shape
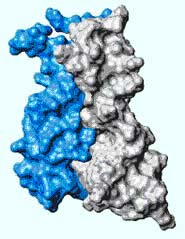
About half of all patients with hereditary breast or ovarian cancer have mutations in a gene called BRCA1. Now the first images of the protein the gene encodes, BRCA1, are helping researchers work out how the mutations cause human disease.
The pictures reveal fine detail of how BRCA1 interacts with other proteins. Such information should help researchers work out how BRCA1 prevents cells becoming cancerous. They suspect that it is involved in DNA repair, controlling cell division and regulating gene activity.
Understanding BRCA1 should also make it easier to design genetic screening programmes to identify individuals at risk and catch cancer early. This is “very important to long-term survival,” says Mark Glover of the University of Alberta in Edmonton, Canada, leader of one of the teams that have solved parts of BRCA1’s structure.
BRCA1 is a big protein — three times the size of haemoglobin, for example. Its chain of 1,863 amino acid links folds into a complex three-dimensional structure. Like a molecular Swiss Army knife, different parts are designed for different jobs.
Most of the mutations associated with breast and ovarian cancers beset the two regions composed of amino acids at the chain ends now under scrutiny. These areas vary least between different species, showing that their function is important enough for natural selection to stamp out slip-ups that would lead to variation.
The ends are probably the most important parts of the molecule, says Richard Baer, a cancer researcher at Columbia University in New York. “They won’t be the whole story, but they’re a big part of the story,” he says.
Marked for death
One end of BRCA1 — the ’N’ terminus — bonds to another protein. The two form a catalyst that joins a small molecule called ubiquitin to other proteins. Ubiquitin tags proteins for destruction — an important stage in the body’s defence against cancer.
This much was already known. Rachel Klevit, of the University of Washington in Seattle, and colleagues have now visualized the interface between BRCA1 and its catalytic collaborator using nuclear magnetic resonance spectroscopy.
Klevit’s team was surprised to find that the collaborator joins to a different part of BRCA1 than they had suspected. The structure shows that mutations can disrupt either this junction or the ubiquitin-attachment machinery.
A full understanding of the N-terminal region’s workings might allow mutant BRCA1 proteins to be repaired, using small molecules to enhance or disrupt the protein machine, says Klevit. Unfortunately the many proteins that interact with BRCA1 make this a daunting task.
“We can do these sorts of things on the chalkboard, but until we’re clear on the multiple functions of all these different proteins it’s going to be difficult,” says Klevit.
Pack up
Glover’s group focused on the other end of the molecule, the ’C’ terminus. Here, around 100 amino acids form structures known as BRCT repeats. These often feature in proteins that repair DNA — a vital part of tumour suppression.
X-ray crystallography reveals that the two BRCT repeats in BRCA1 “pack in a very intimate manner”, says Glover. Mutations that alter the repeats disrupt their packing and unravel the protein. The loss of the last 11 amino acids at the protein’s C terminus is associated with aggressive, early-onset breast cancer.
The structures Klevit’s and Glover’s groups reveal are common to a range of proteins, but between them lies a large stretch of terra incognita, “that doesn’t look like any other protein”, says Klevit. These amino acids — more than 1,500 of them — must do something, she says: “Nature’s not wasteful of its resources.”
letters
Structure of a BRCA1–BARD1 heterodimeric RING–RING complex
PETER S. BRZOVIC, PONNI RAJAGOPAL, DAVID W. HOYT, MARY-CLAIRE KING & RACHEL E. KLEVIT
Nature Structural Biology 8, 833-837 (October 2001)
letters
Crystal structure of the BRCT repeat region from the breast cancer-associated protein BRCA1
R. SCOTT WILLIAMS, RUTH GREEN & J.N. MARK GLOVER
Nature Structural Biology 8, 838-842 (October 2001)
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















