Pseudonymization of patient identifiers for trans-lational research

The usage of clinical patient data for research im-poses risks concerning privacy and informational self-determination of the patient. The gold standard for safeguarding patients' personal rights is the anonymization of the clinical data. However, next-generation-sequencing technologies and various other methods impose ethical constraints that pro-hibit anonymization of patient samples. Individual results from genetic and genomic research should be offered to study participants in a timely manner. A new method for pseudonymizing patient identifi-ers is presented in which the pseudonymization service provider is unable to derive the patient iden-tifier from the pseudonym, but rather this ability is assigned to an authorized third party (Ombuds-man).
Further Information: PDF
DKFZ (German Cancer Research Center, Deutsches Krebsforschungszentrum)
Phone: +49-6221-42 2955
Contact
Dr. Ruth Herzog
Media Contact
All latest news from the category: Technology Offerings
Newest articles

Targeted use of enfortumab vedotin for the treatment of advanced urothelial carcinoma
New study identifies NECTIN4 amplification as a promising biomarker – Under the leadership of PD Dr. Niklas Klümper, Assistant Physician at the Department of Urology at the University Hospital Bonn…
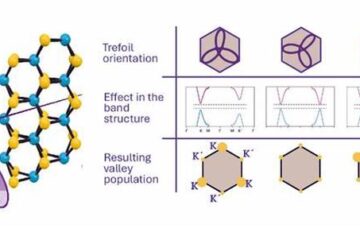
A novel universal light-based technique
…to control valley polarization in bulk materials. An international team of researchers reports in Nature a new method that achieves valley polarization in centrosymmetric bulk materials in a non-material-specific way…

How evolution has optimised the magnetic sensor in birds
The magnetic sense of migratory birds is probably based on the protein cryptochrome 4, and a genetic study has now provided further support for this theory. A team of researchers…

















