When water speaks: solvents make catalysts more efficient
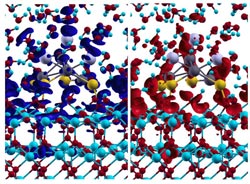
Snapshot of the charge transfer: Water-induced charge transfer at the interface between water and catalyst. The red and blue areas in the left and right image quantify the decrease or increase of the electron density due to the charge transfer at a given time. The blue and red mesh in the lower image section represents the oxide, the grey and yellow balls at the oxide surface the metal. The small blue and red molecules in the upper image section are water molecules.<br>Image: M. Farnesi Camellone, D. Marx<br>
Why certain catalyst materials work more efficiently when they are surrounded by water instead of a gas phase is unclear. RUB chemists have now gleamed some initial answers from computer simulations. They showed that water stabilises specific charge states on the catalyst surface.
“The catalyst and the water sort of speak with each other” says Professor Dominik Marx, depicting the underlying complex charge transfer processes. His research group from the Centre for Theoretical Chemistry also calculated how to increase the efficiency of catalytic systems without water by varying pressure and temperature. The researchers describe the results in the journals “Physical Review Letters” and “Journal of Physical Chemistry Letters.”
Heterogeneous catalysis: water or gas as the second phase
In heterogeneous catalysis, researchers combine substances with two different phases – usually solid and gas. Chemical reactions work faster at the resulting interfaces than without a catalyst. Industry uses heterogeneous catalysis for many processes, for example to transform alcohols into certain aldehydes. Titanium dioxide with gold particles bonded to the surface, for example, is suitable as the solid phase. Water – instead of a gas – as the second phase has several advantages: environmentally harmful substances which are required in traditional procedures for the oxidation of alcohols can easily be replaced by atmospheric oxygen. Also, the whole reaction in water is very efficient, even at moderate temperatures.
Charge transfer between water and catalyst
The theoretical chemists have studied what happens in the catalysis at the molecular level by means of so-called ab initio molecular dynamics simulations. The result: a charge transfer takes place between the water and the catalyst. Electrons, or more specifically portions of electron densities, are moved between the solid and the liquid phase. The researchers speculate that in this way the liquid phase stabilises charge states on the gold surface. The sites where this occurs could be the active centres of the catalyst, where the chemical reactions work efficiently. Unlike water, a gas phase is not able to “talk” to the catalyst in this way, because no charge transfer is possible with the gas phase.
Increasing the efficiency through thermodynamics
In a further study, the team led by Dominik Marx examined a related metal/oxide catalyst of copper and zinc oxide, which is used for the large-scale industrial synthesis of methanol. As the computer simulations showed, especially the interplay between the solid phase and the gas phase is important here for the efficiency. Depending on the pressure and temperature conditions, hydrogen binds to the catalyst surface and thus indirectly stabilises catalytically active centres that occur in this case due to an electron transfer between the metal and the oxide. “Without the hydrogen, put bluntly the centres would not exist”, says Marx. In this way, the thermodynamic conditions in the gas phase put the surface into a certain state which is particularly favourable for the work of the catalyst.
Added value through combination
The two studies thus show that the catalytic efficiency can be controlled both by a solvent and by thermodynamics – namely through the pressure and temperature of the gas phase. However, completely different mechanisms are responsible for this, which the researchers were nevertheless able to elucidate using the same simulation methods. This makes the results directly comparable. In this way, the theorists aim to study in future whether they can improve the copper/zinc oxide system even further by replacing the gas phase with a suitable solvent.
Funding
The chemists at the RUB explore the active role of the solvent in catalytic reactions in the Excellence Cluster “Ruhr Explores Solvation” RESOLV (EXC 1069), which was approved by the German Research Foundation in June 2012.
Bibliographic records
M. Farnesi Camellone, D.Marx (2013). On the Impact of Solvation on a Au/TiO2 Nanocatalyst in Contact with Water, The Journal of Physical Chemistry Letters, doi: 10.1021/jz301891v
L. Martínez-Suárez, J. Frenzel, D. Marx, B. Meyer (2013): Tuning the Reactivity of a Cu/ZnO Nanocatalyst via Gas Phase Pressure, Physical Review Letters, doi: 10.1103/PhysRevLett.110.086108
Further information
Prof. Dr. Dominik Marx, Centre for Theoretical Chemistry, Department of Chemistry and Biochemistry at the Ruhr-Universität, 44780 Bochum, Germany, Tel.+49/234/32-28083, E-mail: dominik.marx@rub.de
Click for more
Theoretical Chemistry at the RUB
http://www.theochem.rub.de/home.en.html
Solvation Science@RUB (RESOLV)
http://www.rub.de/solvation/
Editor: Dr. Julia Weiler
Media Contact
More Information:
http://www.ruhr-uni-bochum.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















