Electrically conductive plastics promising for batteries, solar cells
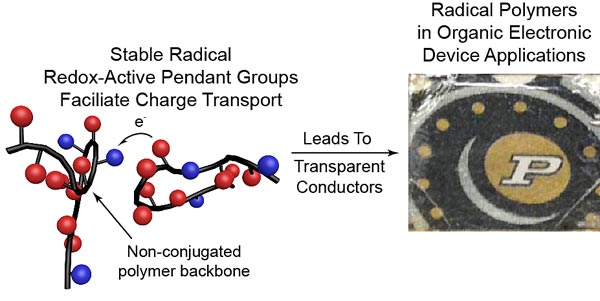
An emerging class of electrically conductive plastics are called "radical polymers.” The graphic at left depicts the structure of a polymer. At right, transparent polymer overlays the Purdue logo. (Purdue University photo)
Researchers have established the solid-state electrical properties of one such polymer, called PTMA, which is about 10 times more electrically conductive than common semiconducting polymers.
“It's a polymer glass that conducts charge, which seems like a contradiction because glasses are usually insulators,” said Bryan Boudouris, an assistant professor of chemical engineering at Purdue University.
The polymer is easy to manufacture, resembling Plexiglas, an inexpensive transparent plastic found in numerous products. However, unlike Plexiglas it conducts electricity.
“We make billions of tons of plastic every year,” Boudouris said. “So imagine if you could produce that same kind of material at that same scale but now it has electronic properties.”
The PTMA is in a class of electrically active polymers that could bring inexpensive transparent solar cells; antistatic and antiglare coatings for cellphone displays; antistatic coverings for aircraft to protect against lightning strikes; flexible flash drives; and thermoelectric devices, which generate electricity from heat.
The polymers have seen commercial use in new types of batteries. However, finding widespread practical applications for the polymers will require increasing the conductivity another 100 to 1,000 times, Boudouris said.
Recent research findings were detailed in a paper published online in May in the journal Macromolecules. A review article on the subject appeared in September in the same journal and is featured on the cover. The American Chemical Society also has recorded a series of podcast with Boudouris, accessible at http://pubs.acs.org/page/mamobx/audio/index.html.
The review article is authored by Purdue graduate students Edward P. Tomlinson and Martha E. Hay, and Boudouris. The research article published in May was authored by graduate student Lizbeth Rostro, undergraduate student Si Hui Wong, and Boudouris.
Polymers are strings of molecules with a central backbone and may contain side chains called “pendant groups” that dangle from the central structure. In radical polymers, it's these pendant groups that allow charge to be transported, conducting current.
To create the radical polymer, the researchers used a procedure called deprotection, which involves replacing a specific hydrogen atom in the pendant group with an oxygen atom, converting it into a so-called radical group.
“We just finally studied deprotection in a way others had not to learn how it affects the electronic properties of the radical polymers,” Boudouris said.
Electrons surround an atom's nucleus in “shells,” and these electrons are usually paired. The oxygen atom in PTMA, however, has one unpaired electron in its outer shell, making it amendable to transporting charge.
“You have to control the deprotection process very well because it makes the conductivity vary by orders of magnitude,” he said.
The researchers have determined that the deprotection step can lead to four distinct chemical functionalities of the radical polymer, two of which are promising for increasing the conductivity of the polymer.
“So manipulating the reaction conditions for this deprotection step, and monitoring closely the resultant chemical functionalities, is critical in tuning the electrical properties of radical polymers,” Boudouris said.
The research is ongoing and has been funded by the National Science Foundation (NSF), the Air Force Office of Scientific Research (AFOSR) and the Defense Advanced Research Projects Agency (DARPA).
Writer: Emil Venere, 765-494-4709, venere@purdue.edu
Source: Bryan Boudouris, 765-496-6056, boudouris@purdue.edu
ABSTRACT
Radical Polymers and Their Application to Organic Electronic Devices
Edward P. Tomlinson , Martha E. Hay , and Bryan W. Boudouris *
School of Chemical Engineering, Purdue University
Macromolecules, 2014, 47 (18), pp 6145–6158
*E-mail: boudouris@purdue.edu
Macromolecules bearing stable radical groups have emerged as extremely useful active materials in organic electronic applications ranging from magnetic devices to flexible batteries. Critical to the success of these open-shell polymers has been the readily tunable nature of their molecular architectures; this important molecular structure–property–performance design paradigm has allowed for significant device performance metrics to be achieved. In this Perspective, the recent advancements regarding the design and device functionality of a common class of open-shell macromolecules, radical polymers, are discussed. Here, radical polymers are defined as macromolecules with nonconjugated carbon backbones, whose optoelectronic functionalities arise due to the presence of stable radical sites on the pendant groups of macromolecular chains. This class of materials provides a unique platform for the design of unique optical and electronic properties in soft materials; however, as with many organic electronic materials, transitioning these gains from the laboratory to the commercial scale remains a primary challenge. As such, we provide context for the significant accomplishments that have been made in the field, describe how these advances have been translated to high-performance devices, and discuss future areas of evaluation for these next-generation polymer electronic materials.
Media Contact
All latest news from the category: Power and Electrical Engineering
This topic covers issues related to energy generation, conversion, transportation and consumption and how the industry is addressing the challenge of energy efficiency in general.
innovations-report provides in-depth and informative reports and articles on subjects ranging from wind energy, fuel cell technology, solar energy, geothermal energy, petroleum, gas, nuclear engineering, alternative energy and energy efficiency to fusion, hydrogen and superconductor technologies.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















