Biocompatible Polymers for Solubilization of Hydrophobic Drugs

Solubilization of hydrophobic drugs is a key issue in pharmaceutical industry. Currently, there are few non-toxic formulations available for drugs like paclitaxel (Taxol® or Abraxane®), which can only solubilize relatively small amounts of active ingredient and may even cause severe side effects in patients.
<p> Superior solubilization of hydrophobic drugs in aqueous solution can be achieved with a novel amphiphilic polymer. The invention utilizes non-toxic polymers for solubilizing hydrophobic drugs by means of defined polymeric micelles. The resulting nanoformulation increases the innate solubility in aqueous solution by 4 orders of magnitude and has a very high loading capacity of up to 40% wt that allows for solubilization of e.g. 8 mg/mL paclitaxel in water. <p> The annual worldwide sales of the few highly hydrophobic drugs that have been successfully solubilized sum up to several billion dollars. The use of this novel carrier system will enable the successful development of numerous APIs into potent drugs, which has an even higher commercial potential. <p> The polymers can be developed into a carrier for paclitaxel and a multitude of other hydrophobic drugs for parenteral administration. The novel polymer system has been tested with several compounds like paclitaxel, cyclosporine or amphotericin and can be tailored to optimize properties for the solubilization of various other drugs and active agents.
Further Information: PDF
Bayerische Patentallianz GmbH
Phone: +49 89 5480177-0
Contact
Peer Biskup
Media Contact
All latest news from the category: Technology Offerings
Newest articles
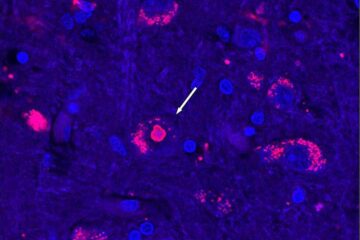
After 25 years, researchers uncover genetic cause of rare neurological disease
Some families call it a trial of faith. Others just call it a curse. The progressive neurological disease known as spinocerebellar ataxia 4 (SCA4) is a rare condition, but its…
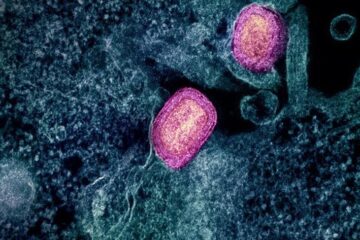
Lower dose of mpox vaccine is safe
… and generates six-week antibody response equivalent to standard regimen. Study highlights need for defined markers of mpox immunity to inform public health use. A dose-sparing intradermal mpox vaccination regimen…

Efficient, sustainable and cost-effective hybrid energy storage system for modern power grids
EU project HyFlow: Over three years of research, the consortium of the EU project HyFlow has successfully developed a highly efficient, sustainable, and cost-effective hybrid energy storage system (HESS) that…

















