Rutgers Chemists Develop Technology to Produce Clean-Burning Hydrogen Fuel
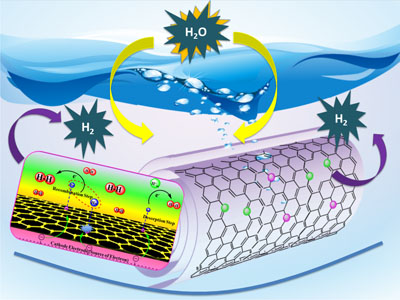
Image: Tewodros Asefa A new technology based on carbon nanotubes promises commercially viable hydrogen production from water.
Rutgers researchers have developed a technology that could overcome a major cost barrier to make clean-burning hydrogen fuel – a fuel that could replace expensive and environmentally harmful fossil fuels.
The new technology is a novel catalyst that performs almost as well as cost-prohibitive platinum for so-called electrolysis reactions, which use electric currents to split water molecules into hydrogen and oxygen. The Rutgers technology is also far more efficient than less-expensive catalysts investigated to-date.
“Hydrogen has long been expected to play a vital role in our future energy landscapes by mitigating, if not completely eliminating, our reliance on fossil fuels,” said Tewodros (Teddy) Asefa, associate professor of chemistry and chemical biology in the School of Arts and Sciences. “We have developed a sustainable chemical catalyst that, we hope with the right industry partner, can bring this vision to life.”
Asefa is also an associate professor of chemical and biochemical engineering in the School of Engineering.
He and his colleagues based their new catalyst on carbon nanotubes – one-atom-thick sheets of carbon rolled into tubes 10,000 times thinner than a human hair.
Finding ways to make electrolysis reactions commercially viable is important because processes that make hydrogen today start with methane – itself a fossil fuel. The need to consume fossil fuel therefore negates current claims that hydrogen is a “green” fuel.
Electrolysis, however, could produce hydrogen using electricity generated by renewable sources, such as solar, wind and hydro energy, or by carbon-neutral sources, such as nuclear energy. And even if fossil fuels were used for electrolysis, the higher efficiency and better emissions controls of large power plants could give hydrogen fuel cells an advantage over less efficient and more polluting gasoline and diesel engines in millions of vehicles and other applications.
In a recent scientific paper published in Angewandte Chemie International Edition, Asefa and his colleagues reported that their technology, called “noble metal-free nitrogen-rich carbon nanotubes,” efficiently catalyze the hydrogen evolution reaction with activities close to that of platinum. They also function well in acidic, neutral or basic conditions, allowing them to be coupled with the best available oxygen-evolving catalysts that also play crucial roles in the water-splitting reaction.
The researchers have filed for a patent on the catalyst, which is available for licensing or research collaborations through the Rutgers Office of Technology Commercialization. The National Science Foundation funded the research.
Asefa, an expert in inorganic and materials chemistry, joined the Rutgers faculty in 2009 after four years as an assistant professor at Syracuse University. Originally from Ethiopia, he is a resident of Montgomery Township, N.J. In addition to catalysis and nanocatalysis, his research interests include novel inorganic nanomaterials and nanomaterials for biological, medical biosensing and solar cell applications.
For more information, please contact Carl Blesch of Rutgers Media Relations at 848-932-0550 or cblesch@ucm.rutgers.edu
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Webb captures top of iconic horsehead nebula in unprecedented detail
NASA’s James Webb Space Telescope has captured the sharpest infrared images to date of a zoomed-in portion of one of the most distinctive objects in our skies, the Horsehead Nebula….
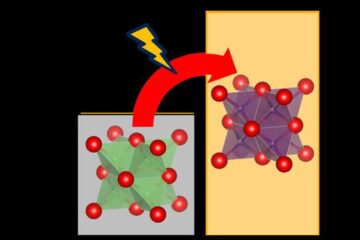
Cost-effective, high-capacity, and cyclable lithium-ion battery cathodes
Charge-recharge cycling of lithium-superrich iron oxide, a cost-effective and high-capacity cathode for new-generation lithium-ion batteries, can be greatly improved by doping with readily available mineral elements. The energy capacity and…
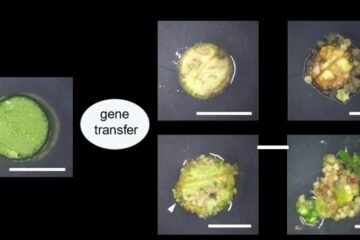
Novel genetic plant regeneration approach
…without the application of phytohormones. Researchers develop a novel plant regeneration approach by modulating the expression of genes that control plant cell differentiation. For ages now, plants have been the…





















