Drop in found out
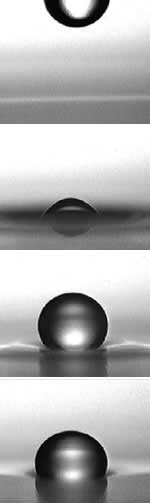
Long drop: soap and air keep water afloat on water. <br>© Y. Amarouchene
Air lets water droplets skim across the kitchen sink.
Scientists have found the answer to a question pondered over many a kitchen sink: why do little droplets skim across the surface of washing-up water rather than mix with it?
Yacine Amarouchene and colleagues at the University of Bordeaux in Talence, France have discovered that the height from which the drops fall has no effect on their lifespan1.
Soap, detergent – and indeed food grease – are ’surfactants’. They form a kind of skin on the surface of water that stabilizes a droplet, preventing it from merging where it rests. Droplets of pure water coalesce in a few thousandths of a second; when they contain a little surfactant they last hundreds of times longer.
The interest in coalescing droplets goes beyond the kitchen sink. When applying thin coats of a liquid as a spray, for example in car-painting, droplets need to merge quickly and smoothly at the surface of the wet film. Emulsions and foams, meanwhile, are sustained by inhibiting or slowing the coalescence of droplets or bubbles. Amarouchene’s group hope their theory might help chemical engineers and technologists to promote or prevent the effect, as required.
Droplets containing soap or detergent that fall a short distance onto a water surface bounce as if on a trampoline, the researchers found. The droplets then rest in a slight dip with a very thin film of air separating the two water surfaces.
How long the droplet lasts depends on how quickly the air is squeezed out from this interface, which in turn depends on the concentration of surfactants at the droplet surface. For pure water, the air film can thin very fast. A skin of surfactants at the water surface makes it harder for air to flow past. Air flow deforms this skin; and deformed skin slows the flow further.
The researchers find that, for a droplet of a particular size and containing a particular amount of surfactant, there is a characteristic residence time on the surface. Dropping them from increasing heights simply increases the chance of rupture on impact – it doesn’t alter the average lifetime of the drops that survive.
References
- Amarouchene, Y., Cristobal, G. & Kellay, H. Noncoalescing drops. Physical Review Letters, 87, 206104 (2001).
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Faster, more energy-efficient way to manufacture an industrially important chemical
Zirconium combined with silicon nitride enhances the conversion of propane — present in natural gas — needed to create in-demand plastic, polypropylene. Polypropylene is a common type of plastic found…

Energy planning in Ghana as a role model for the world
Improving the resilience of energy systems in the Global South. What criteria should we use to better plan for resilient energy systems? How do socio-economic, technical and climate change related…

Artificial blood vessels could improve heart bypass outcomes
Artificial blood vessels could improve heart bypass outcomes. 3D-printed blood vessels, which closely mimic the properties of human veins, could transform the treatment of cardiovascular diseases. Strong, flexible, gel-like tubes…




















