Bugs offer power tips
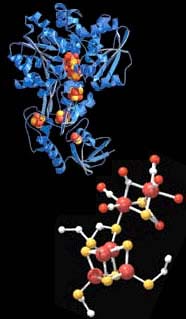
Hydrogenases could fuel the future. <br>© J. Peters <br>
Chemists copy bacterial tricks for making clean fuel.
Bacteria are teaching chemists their tips for creating lean, green fuel. US researchers have developed a catalyst based on a bacterial enzyme that converts cheap acids to hydrogen, the ultimate clean power source.
Unlike other fuels, hydrogen is non-polluting: its combustion makes only water, instead of greenhouse gas carbon dioxide or the poison carbon monoxide. Thomas Rauchfuss and colleagues at the University of Illinois at Urbana-Champaign believe they can steal the secrets of hydrogen-generating bacteria to make the gas cheaply and efficiently1.
Such bacteria contain enzymes called hydrogenases, which can make hydrogen gas from acids. Rauchfuss and his team made a synthetic catalyst that efficiently mimics this enzyme. For industrial hydrogen production, such catalysts might be easier to make, modify and maintain compared to living cells. Thus it should be possible to extract fuel from inexpensive, plentiful acids, they hope.
Like natural gas, hydrogen can be burnt and the energy converted directly into electricity in power sources called fuel cells. Prototypes of hydrogen-powered vehicles have been made, but availability of hydrogen is a sticking point. Although it can be made from sea water by electrolysis, this is not economical.
But hydrogen production and breakdown are a standard part of the metabolism of some bacteria in which they help to convert carbon dioxide and nitrogen into biologically useful compounds. Present-day hydrogen-producing bacteria are thought to be similar to those that predominated during the early days of life on Earth, when carbon dioxide and nitrogen are believed to have been major constituents of the atmosphere.
There are two general classes of hydrogenases. In one, the ’active site’ in the enzyme responsible for hydrogen conversion contains a nickel atom and an iron atom; in the other, this site contains two iron atoms. The two iron atoms are linked by a chemical bond, and are attached to other chemical groups including cyanide, carbon monoxide and sulphur-containing groups. The whole ’core’ is wrapped up in a protein coat. The team developed a small molecule that mimics the ’naked’ core of the active site, minus the coat.
The researchers are confident that it should be possible to make a version that dissolves in water, which would be industrially more useful. At present the catalyst dissolves only in organic solvents.
References
- Gloaguen, F., Lawrence, J. D. & Rauchfuss, T. B. Biomimetic hydrogen evolution catalyzed by an iron carbonyl thiolate. Journal of the American Chemical Society, 123, 9476 – 9477, (2001).
Media Contact
More Information:
http://www.nature.com/nsu/011011/011011-3.htmlAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Faster, more energy-efficient way to manufacture an industrially important chemical
Zirconium combined with silicon nitride enhances the conversion of propane — present in natural gas — needed to create in-demand plastic, polypropylene. Polypropylene is a common type of plastic found…

Energy planning in Ghana as a role model for the world
Improving the resilience of energy systems in the Global South. What criteria should we use to better plan for resilient energy systems? How do socio-economic, technical and climate change related…

Artificial blood vessels could improve heart bypass outcomes
Artificial blood vessels could improve heart bypass outcomes. 3D-printed blood vessels, which closely mimic the properties of human veins, could transform the treatment of cardiovascular diseases. Strong, flexible, gel-like tubes…




















