Data effort improves flow toward ’greener’ chemistry
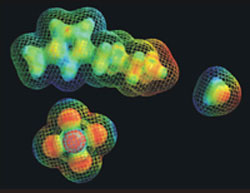
Molecular "space filling" models demonstrate the difference in size for the positively charged "anion" (top image) and the negatively charged "cation" (bottom left) that combine to form a promising ionic liquid. It is still a mystery how the much smaller water molecule (right) can have such a large effect on the viscosity of such ionic liquids.
Jeopardy answer: Death Valley and “ionic liquids.” Correct question: Where does a little bit of water make a whole lot of difference?
Scientists at the National Institute of Standards and Technology (NIST) report* that flow properties for a relatively new class of alternative solvents called ionic liquids are extremely sensitive to tiny amounts of water. For example, for one of these solvents, just a 0.01 percent increase in water dissolved into a sample, caused a 1 percent decrease in flow resistance–a 100-fold effect. The finding should be helpful in the design of new industrial processes such as chemical separations that are both more efficient and more environmentally friendly.
Ionic liquids are salts. Just like table salt, ionic liquids consist of two components, one positively and one negatively charged. Unlike most simple salts, however, most of these new solvents are liquid at room temperature.
“People in industry are very interested in using ionic liquids because unlike most organic solvents, they don’t evaporate and they are not flammable,” explains NIST’s Jason Widegren, lead author on the paper.
However, before ionic fluids can be used widely in industrial processes, reliable property data on characteristics like flow resistance (viscosity), density and thermal conductivity must be collected.
The new data help explain why reproducible measurements of viscosity for ionic liquids have been very difficult to achieve and published results have differed by 30 percent or more. Even the slightest contamination of samples with water vapor absorbed from the air dramatically affects measurements. The NIST group avoided these problems by carefully drying their samples and measuring water content both before and after each viscosity measurement.
The NIST work is part of a larger effort, conducted in conjunction with the International Union of Pure and Applied Chemistry, to perform “round robin” thermophysical property testing on the most promising ionic fluids and make the resulting data available to the scientific community.
Media Contact
More Information:
http://www.nist.govAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















