Mikado in the cell
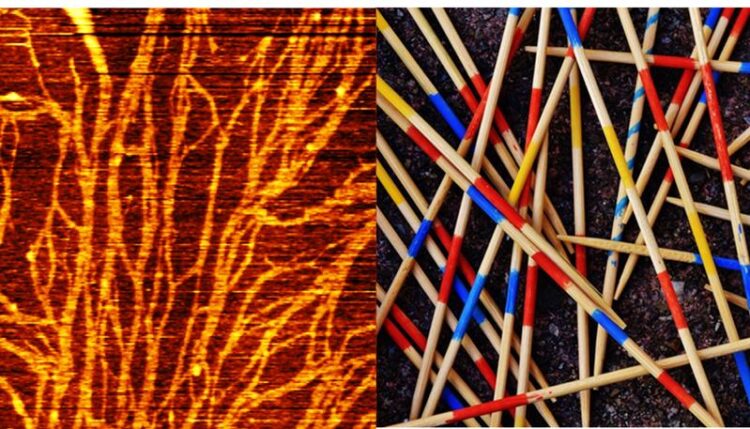
FUS proteins form interconnected "Mikado networks". These could be a building block in the development of neurodegenerative diseases.
© MPI for Polymer Research
Arrangement of proteins could be responsible for diseases.
Parkinson’s, Alzheimer’s or Huntington’s disease: the behavior of certain molecules that play a role in sub-cellular processes influence the development of such neurodegenerative diseases. Scientists from Mischa Bonn’s department at the Max Planck Institute for Polymer Research and Sapun Parekh’s lab at the University of Texas have now studied a specific protein using various methods to better understand the mechanism behind these diseases.
Processes inside human cells are tightly regulated in time and space by various enzymes and proteins. However, if processes become unbalanced – for example, because cells experience increased stress – these processes can also lead to diseases.
For example, proteins can “aggregate” – that is, cluster together and form extended, ordered, straight fibers, similar to a Mikado. While most proteins have a well-defined three-dimensional structure, some exist in cells without any structure, like a long string. This category of proteins is called intrinsically disordered. Recently such intrinsically disordered proteins have received considerable attention as driving cellular organization and have been linked to neurodegeneration. However, it is unclear how these disordered and flexible proteins become structured to build the Mikado.
A team of researchers at the Max Planck Institute for Polymer Research and the University of Texas has now shown that interfaces can trigger aggregation of a model intrinsically disordered protein – called FUS (fused in sarcoma). This disordered protein is flexible in the bulk, but at a hydrophobic interface, it forms fibers. These FUS proteins form connected “Mikado-networks ” that cannot be easily broken down and may contribute to the development of neurodegenerative diseases.
“We looked at the formation of FUS fibers at the hydrophobic interface using laser-based methods including spectroscopy and microscopy,” say Mischa Bonn and Yuki Nagata. The researchers observed the formation of fibers through the collective ordered assembly of intrinsically disordered proteins. The researchers further showed that protein mobility was dramatically reduced upon fiber formation: the proteins are stuck in the fibers they form.
“We were able to demonstrate that hydrophobic interfaces – for example, small oily droplets in cells – can seed molecular ordering and fiber formation,” explains Sapun Parekh, also a group leader in Mischa Bonn’s department and a professor at the University of Austin, Texas. “This formation happens at surprisingly low concentrations: concentrations 600 times lower than necessary for forming loose protein clusters in solution,” Parekh adds.
The scientists hope their research will contribute to future understanding of how neurodegenerative diseases develop. They have now published their study in the journal Nature Chemistry.
Wissenschaftliche Ansprechpartner:
Dr. Yuki Nagata
nagata@mpip-mainz.mpg.de
Prof. Dr. Mischa Bonn
bonn@mpip-mainz.mpg.de
Prof. Dr. Sapun Parekh
parekh@mpip-mainz.mpg.de
Originalpublikation:
Daria Maltseva, Sayantan Chatterjee, Chun-Chieh Yu, Mateusz Brzezinski, Yuki Nagata, Grazia Gonella, Anastasia C. Murthy, Jeanne C. Stachowiak, Nicolas L. Fawzi, Sapun Parekh & Mischa Bonn: Fibril formation and ordering of disordered FUS LC driven by hydrophobic interactions; DOI: 10.1038/s41557-023-01221-1
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















