Converting methane to methanol
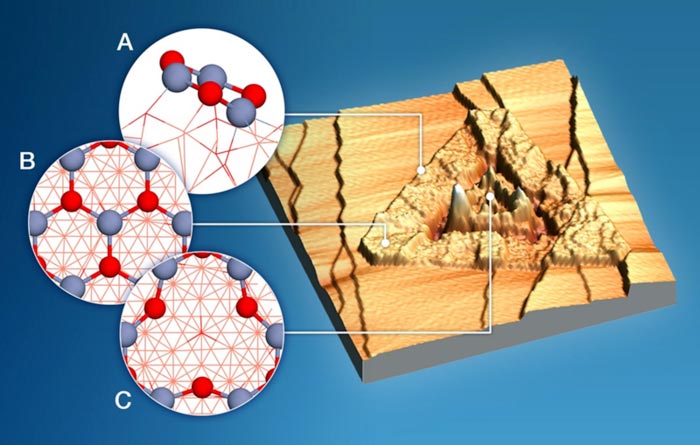
A scanning tunneling electron microscope image of a copper-zinc oxide catalyst that converts methane to methanol with and without water. Insets show the molecular structures of three different phases (blue is zinc, red is oxygen).
Image courtesy of Brookhaven National Laboratory. A version appears in Huang E., et al., Selective Methane Oxidation to Methanol on ZnO/Cu2O/Cu(111) Catalysts: Multiple Site-Dependent Behaviors. Journal of the American Chemical Society 143, 45 (2021)
– with and without water.
Studies of a common catalyst suggest strategies for improving the conversion of a natural gas component to useful chemicals.
The Science
Chemists have been searching for efficient catalysts—substances that speed up chemical reactions—to convert methane into methanol. Methane, a major component of abundant natural gas, is sometimes flared off as waste at wells. Methanol is an easily transported liquid fuel and a building block for making other valuable chemicals. Adding water to the methane conversion reaction can help produce methanol, but it also complicates the process for industry. Now scientists have identified a common industrial catalyst that completes the conversion along different pathways depending on whether water is present or not. The findings suggest strategies for improving catalysts for the less-complicated, water-free reaction.
The Impact
Scientists have used water to get the methane to methanol reaction going because it improves selectivity—the ability to drive the reactants to a specific desired product. Water also makes it easier to extract the methanol. But water adds complexity and cost to the process. At the temperatures and in the amounts required for this reaction, water exists as large quantities of steam. This steam must be controlled when the reaction is used in an industrial setting. Finding a catalyst to complete the reaction without water would simplify the synthesis of methanol from waste methane. An efficient process could potentially reduce the amount of methane, a potent greenhouse gas, by turning it into useful products.
Summary
In a recent study looking at a different catalyst for converting methane to methanol, scientists discovered that adding water improves selectivity. It keeps the reaction from running away to fully oxidize methanol to carbon monoxide (CO) and carbon dioxide (CO2). In this new study, a team from Brookhaven National Laboratory (BNL), Lawrence Berkley National Laboratory (LBNL), Stony Book University, and the Universidad Central de Venezuela tested a different catalyst to see if it could achieve this selectivity without water. Copper-zinc oxide, an affordable and readily available catalyst, had the best selectivity of any catalyst tested for methanol production without water, and even higher selectivity with water. The team used X-rays to study the catalyst under different reaction conditions at the Advanced Light Source (ALS), a Department of Energy (DOE) user facility at LBNL. X-ray-generated “chemical fingerprints” tracked methanol formation and intermediates. The scientists also used computing resources at BNL’s Center for Functional Nanomaterials (CFN) and the National Energy Research Scientific Computing Center (NERSC) at LBNL, both DOE user facilities, to identify the active sites on the catalyst and model the kinetics of different reaction pathways. Together, the data indicate that the reaction proceeds along two different pathways—one with and one without water—using two different sites of the catalyst. Additional modeling studies will now test other catalytic compositions, aiming to further improve water-free conversion of methane to methanol.
Funding
This research was funded by the DOE Office of Science, Biological and Environmental Research program. The ALS, CFN, and NERSC are DOE Office of Science user facilities.
Media Contact
Michael Church
DOE/US Department of Energy
michael.church@science.doe.gov
Office: 2028416299
https://www.energy.gov/science/bes/articles/converting-methane-methanol-and-without-water
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















