Cheap alloy rivals expensive platinum to boost fuel cells
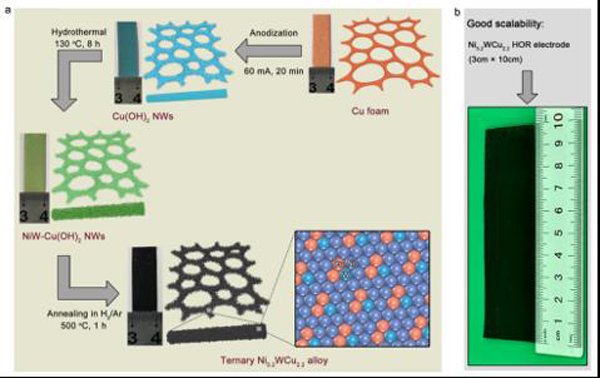
Synthesis diagram of Ni5.2WCu2.2 and a Ni5.2WCu2.2 electrode of size 3×10cm2 obtained in this way.
Credit: QIN Shuai et al.
As the cleanest renewable energy, hydrogen energy has attracted special attention in the research. Yet the commercialization of traditional proton exchange membrane fuel cells (PEMFCs), which consume hydrogen and produce electricity, is seriously restricted due to the chemical reaction of PEMFCs cathode largely relies on expensive platinum-based catalysts.
A solution is to change the acidic electrolyte of PEMFCs to alkaline. Such fuel cells are called anion exchange membrane fuel cells (AEMFCs), and they allow for the use of cheaper metal elements like Co, Ni or Mn to design electrocatalysts.
The research team led by Prof. GAO Minrui from University of Science and Technology of China (USTC) followed this solution and developed a practical and scalable way to manufacture a novel Ni-W-Cu alloy, Ni5.2WCu2.2, as the cathode for AEMFCs. The result was published on Nature Communications.
The team first grew Cu(OH)2 nanowires from a three-dimensional foam copper skeleton by anodic oxidation. The obtained nanowires were then immersed in a solution containing Ni and W elements. After hydrothermal synthesis and annealing, the Ni-W-Cu alloy is produced.
The ternary Ni5.2WCu2.2 alloy can catalyze the oxidation of hydrogen in alkaline medium 4.31 times more efficient than the benchmark platinum/carbon anode.
It has an oxidation potential as high as 0.3V in comparison with the reversible hydrogen electrode and can maintain high activity for up to 20h under such overpotential, proceeding anodes based on non-platinum-group metals.
The alloy catalyst also showed excellent resistance to CO poisoning, and maintained high activity in 20000 ppm CO/H2 mixed atmosphere.
Analysis showed that the projected density of states of Ni5.2WCu2.2 alloy lies in the lowest at Fermi level, which indicates that the alloy has the optimal hydrogen binding energy. The multiple-element alloying effect renders the Ni-based alloy a high activity catalyst and offers oxidation resistance.
This work sheds light on further exploration of multiple-element alloys composed of cheap metals, thereby aiding the development of more efficient hydrogen oxidation catalysts for AEMFC anodes.
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















