A new approach to recording cellular activities
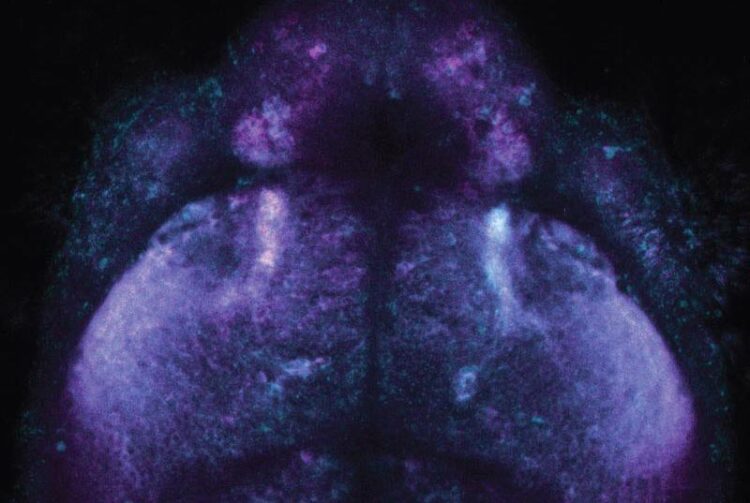
Inside a fish brain: the novel chemical labeling method makes use of distinguishable fluorescent dyes, here magenta and blue, to record cellular activities for later analysis.
(c) MPI for Medical Research
In living cells, a vast number of transient events occur simultaneously, each of them important for a given cell in carrying out its function. The faithful recording of these transient activities is a prerequisite for a molecular understanding of life, yet obtaining such recordings is extremely challenging. Scientists at the Max Planck Institute for Medical Research in Heidelberg and their collaboration partners have created a novel technology that allows cellular events to be recorded through chemical labeling with fluorescent dyes for later analysis, opening up completely new ways to study cellular physiology. This new method has now been published in Science.

Clearly visible, fast, later analysis possible: color-coded cell activity in a fly brain. (c) MPI for Medical Research
The recording of transient cellular events plays a decisive role in examining and understanding biological processes, yet it presents significant technical challenges. An ideal recording method would observe large populations of cells simultaneously, would work in the test tube and in living animals, and would allow the recorded observations to be retrieved and analyzed at a later time. So far, methods that meet these criteria have been largely lacking: a gap that the new technology could now bridge.
Recorder proteins can be irreversibly marked
“Our technology is based on a recorder protein which becomes irreversibly labeled with a fluorescent dye when an event of interest occurs in its vicinity”, explains Magnus-Carsten Huppertz, postdoctoral researcher in the Department of Chemical Biology at the MPI for Medical Research. “This enables scientists to study very large numbers of cells in parallel ̶ in vivo or in vitro.”
Distinguishable substrates record successive periods of activity
The team, led by Kai Johnsson and Julien Hiblot, designed proteins that become labeled when a specific cellular activity and a fluorescent substrate are simultaneously present. The washing-in and the washing-out of the substrate defines the recording period, while the cellular activity determines the degree of the labeling. Moreover, by using distinguishable substrates, different phases within a period of activity can be recorded.
In their studies, constructed recorders for three different processes of central interest: receptor activation, protein-protein interactions and changes in calcium ion (Ca2+) concentration. The latter was employed to study the heterogeneity of Ca2+ changes in cellular networks derived from glioblastoma, an aggressive brain tumor. In close collaboration with the groups of Lisa Fenk and Herwig Baier at the Max Planck Institute for Biological Intelligence in Martinsried, the authors successfully recorded patterns of neuronal activity in flies and zebrafish.
“In the end, we have developed such a versatile recording platform for the parallel analysis of numerous simultaneous transient cellular events in vitro and in vivo“, concludes Jonas Wilhelm, postdoctoral researcher at in the same department. The scientists present their approach and their findings in the latest issue of Science.
Tested in complex experimental settings
The main challenge the scientists faced during their work was to refine the newly developed recorder platform to ensure its robustness and efficient performance across a range of biological model systems. To explore the use of this new technology under various conditions, they established a variety of composite experimental arrangements.
Potential for accelerating research
“We are excited to provide new molecular tools with the potential to enable new types of experiments and accelerate research across different fields such as neurobiology and oncology”, say Magnus-Carsten Huppertz and Jonas Wilhelm. “We were lucky to be able to collaborate with scientists from different disciplines to make this new technology possible.” In addition to the Max Planck Institute for Biological Intelligence, scientists at the German Cancer Research Center (DKFZ), the National Center for Tumor Diseases (NCT), Heidelberg University, Janelia Research Campus, Virginia, USA, and École Polytechnique Fédérale de Lausanne (EPFL), Switzerland contributed to the work.
Wissenschaftliche Ansprechpartner:
Prof. Dr. Kai Johnsson
Chemical Biology Department, Max Planck Institute for Medical Research
kai.johnsson@mr.mpg.de
Originalpublikation:
Recording physiological history of cells with chemical labeling
Magnus-Carsten Huppertz, Jonas Wilhelm, Vincent Grenier, Martin W. Schneider,
Tjalda Falt, Nicola Porzberg, David Hausmann, Dirk C. Hoffmann, Ling Hai,
Miroslaw Tarnawski, Gabriela Pino, Krasimir Slanchev, Ilya Kolb, Claudio Acuna, Lisa
M. Fenk, Herwig Baier, Julien Hiblot, Kai Johnsson
Science
DOI: 10.1126/science.adg0812
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















