Shoe Strings and Egg Openers – Max Planck Scientists Discover Photosynthesis Helper Protein
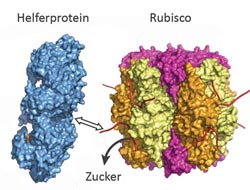
The helper protein (blue) pulls at one end of Rubisco (multicolored) and thus, releases the sugar. The blockade is lifted. Graphic: Manajit Hayer-Hartl / Copyright: Max Planck Institute of Biochemistry<br>
Red algae, in contrast, use a slightly different mechanism and are thus more productive. Scientists from the Max Planck Institute of Biochemistry (MPIB) in Martinsried near Munich have now identified a so far unknown helper protein for photosynthesis in red algae. “We could elucidate its structure and its intriguing mechanism,” says Manajit Hayer-Hartl, MPIB group leader. “Comparing its mechanism to the one in green plants could help to design more efficient plants.” Their work has led to two recent publications in Nature and Nature Structural & Molecular Biology.
Green plants, algae and plankton metabolize carbon dioxide (CO2) and water into oxygen and sugar in the presence of light. Without this process called photosynthesis, today’s life on earth would not be possible. The key protein of this process, called Rubisco, is thus one of the most important proteins in nature. It bonds with carbon dioxide and starts its conversion into sugar and oxygen.
„Despite its fundamental importance, Rubisco is an enzyme fraught with shortcomings“, says Manajit Hayer-Hartl, head of the Research Group “Chaperonin-assisted Protein Folding” at the MPIB. One of the problems is that Rubisco binds to wrong sugar molecules that inhibit its activity. The inhibitors have to be removed by a special helper protein, called Rubisco activase. The Max Planck scientists now discovered that during evolution two different Rubisco activases developed in plants and in red algae. They differ in structure and in their working mechanism.
Two Ways of Restoring Activity
The newly discovered Rubisco activase in red algae repairs useless Rubisco proteins by pulling on one end of the protein, like someone who opens a shoe string. In doing so, the helper protein opens the active center of Rubisco and releases the inhibitory sugar. The respective Rubisco activase in green plants works more like an egg opener, squeezing the inactive Rubisco protein and forcing it to let go off the sugar molecules. “Understanding the structure and function of the two activase helper proteins should facilitate efforts in biotechnology to generate plants and microorganisms that are able to convert more CO2 into valuable biomass than nature does,” hopes Manajit Hayer-Hartl.
Original Publications:
O. Mueller-Cajar, M. Stotz, P. Wendler, F. U. Hartl, A. Bracher & M. Hayer-Hartl: Structure and function of the AAA1protein CbbX, a red-type Rubisco activase. Nature, November 2, 2011
M. Stotz, O. Mueller-Cajar, S. Ciniawsky, P. Wendler, F. U. Hartl, A. Bracher & M. Hayer-Hartl: Structure of green-type Rubisco activase from tobacco. Nature Structural & Molecular Biology, November 6, 2011
Contact:
Dr. Manajit Hayer-Hartl
Chaperonin-assisted Protein Folding
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
mhartl@biochem.mpg.de
Anja Konschak
Public Relations
Max Planck Institute of Biochemistry
An Klopferspitz 18
82152 Martinsried
Phone ++49/89-8578-2824
E-mail: konschak@biochem.mpg.de
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















