Scientists discover how proteins form crystals that tile a microbe's shell
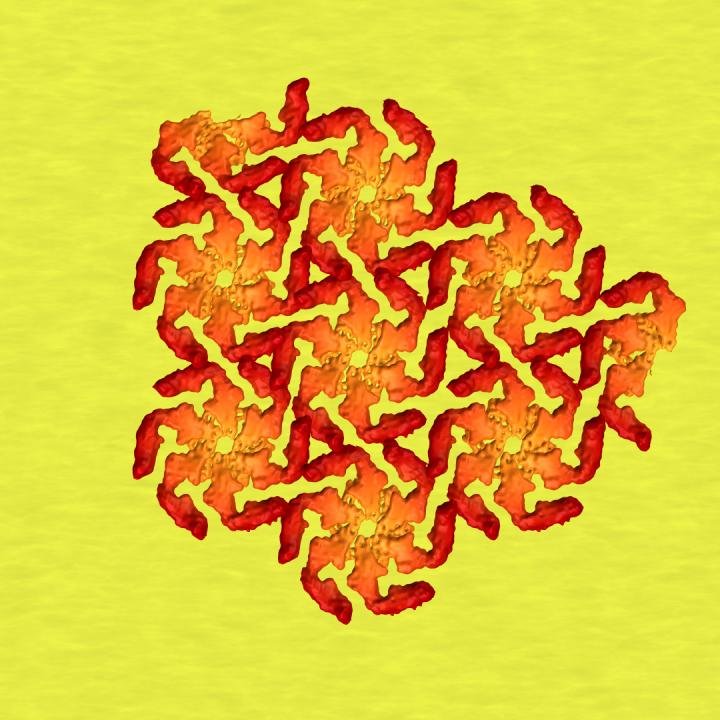
In this illustration, protein crystals join six-sided 'tiles' forming at top left and far right, part of a protective shell worn by many microbes. A new study zooms in on the first steps of crystal formation and helps explain how microbial shells assemble themselves so quickly. Credit:Greg Stewart/SLAC National Accelerator Laboratory
Many microbes wear beautifully patterned crystalline shells, which protect them from a harsh world and can even help them reel in food.
Studies at the Department of Energy's SLAC National Accelerator Laboratory and Stanford University have revealed this food-reeling process and shown how shells assemble themselves from protein building blocks.
Now the same team has zoomed in on the very first step in microbial shell-building: nucleation, where squiggly proteins crystallize into sturdy building blocks, much like rock candy crystallizes around a string dipped into sugar syrup.
The results, published today in the Proceedings of the National Academy of Sciences, could shed light on how the shells help microbes interact with other organisms and with their environments, and also help scientists design self-assembling nanostructures for various tasks.
Jonathan Herrmann, a graduate student in Professor Soichi Wakatsuki's group at SLAC and Stanford, collaborated with the structural molecular biology team at SLAC's Stanford Synchrotron Radiation Lightsource (SSRL) on the study.
They scattered a powerful beam of X-rays off protein molecules that were floating in a solution to see how the atomic structures of the molecules changed as they nucleated into crystals. Meanwhile, other researchers made a series of cryogenic electron microscope (cryo-EM) images at various points in the nucleation process to show what happened over time.
They found out that crystal formation takes place in two steps: One end of the protein molecule nucleates into crystal while the other end, called the N-terminus, continues to wiggle around. Then the N-terminus joins in, and the crystallization is complete. Far from being a laggard, the N-terminus actually speeds up the initial nucleation step - although exactly how it does this is still unknown, the researchers said – and this helps explain why microbial shells can form so quickly and efficiently.
###
Some of the X-ray data was collected at Lawrence Berkeley National Laboratory's Advanced Light Source, which like SSRL is a DOE Office of Science user facility. Researchers from the University of British Columbia and from Professor Lucy Shapiro's laboratory at Stanford also contributed to this work, funded in part by the National Institute of General Medical Sciences and the Chan Zuckerberg Biohub. Work in Wakatsuki's labs at SLAC and Stanford was funded by a Laboratory Directed Research and Development grant from SLAC, the DOE Office of Science's Office of Biological and Environmental Research, and Stanford's Precourt Institute for Energy.
Citation: Jonathan Herrmann et al., PNAS, 17 December 2019 (10.1073/pnas.1909798116)
SLAC is a vibrant multiprogram laboratory that explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by scientists around the globe. With research spanning particle physics, astrophysics and cosmology, materials, chemistry, bio- and energy sciences and scientific computing, we help solve real-world problems and advance the interests of the nation.
SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















