Pulsating chemistry
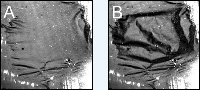
A shows the catalytic foil in a relatively smooth state, image B shows a state with many folds. <br>Photo: Fritz-Haber-Institut <br>
Researchers at the Fritz-Haber Institute in Berlin have recently discovered chemical-thermal-mechanical oscillations that show, indirectly, the rate of certain reactions.
The pattern formation of a catalytic surface reaction is influenced by the temperature at which the reaction takes place. If the temperature of the surface is changed, then the course of the chemical processes changes as well. In extreme cases this change can lead to front formation, i.e. patterns, or, for example, to the overheating of the catalysts. Scientists in the research group led by Professors Harm Hinrich Rotermund and Gerhard Ertl at the Fritz-Haber Institute in Berlin have recently begun to study these processes.
In particular, they have investigated more precisely the influence of heat production during catalytic surface reactions between oxygen and carbon monoxide and pattern formations using an ultra-thin platinum catalyst. During the investigation it was established that, like a beating heart, the platinum foil began to pulsate mechanically during the reactions. With mathematical models and computer simulations the scientists were able to show that the elastic deformations of the foil were in fact due to the oscillation of the chemical reaction itself. This effect can now be used to precisely measure the amount of heat created during these chemical reactions (Science, 20 June, 2003).
Initially, the goal of the researchers (G. Ertl, H.H. Rotermund M. Schunack, Jp. Wolff) was merely investigating the influence of the reaction-induced heat on the pattern formation (spiral waves, standing waves, solitons, etc.) in catalytic surface reactions. The heating of a platinum catalyst during the creation of carbon dioxide is hardly measurable under normal conditions. This means working at a constant temperature with very small pressures (on the order of one-millionth of the normal air pressure) of oxygen and carbon monoxide, the experimental gases which are used, and a sample thickness of approximately 1 mm. In order to be able to fully investigate the temperature effects in spite of the difficulties in measuring, an ultra-thin (0.0002 Millimeter) platinum foil was used as the catalyst. The thickness of the foil is crucial because the heat from the reaction can not be conducted away or neutralized by the metal.
The heat pattern of the reaction is observed using a highly sensitive infrared camera. The resulting pictures clearly showed how, during the reaction, the temperature of the ultra-thin foil fluctuated by several degrees Celsius. And, with only a slightly rise in pressure, temperature oscillations of as much as 20 to 30 degrees were observed. The infrared pictures looked surprisingly like the entire foil catalyst was mechanically oscillating and folds were appearing. An explanation finally came with the use of a normal camera: Every three to four seconds the self-supporting foil was drastically deformed. Two pictures of these oscillations are shown in the figure below.
Figure
The underlying reasons for the oscillations remained, however, unclear. The temperature of the thin foil was obviously increased from the heat of the reaction. This rise in temperature then accelerated the reaction, causing the temperature to go up more. At some point it would be expected that no further increase in the temperature would be found because the additional heat from the reaction would equal the heat lost via radiation. What is observed, however, is a sudden and rapid decrease of the temperature, after which the foil becomes once again smooth.
While Prof. I.G. Kevrekidis visited the Fritz-Haber-Institut extensive discussions lead to further investigations of this phenomenon. Additional experiments were carried out in which the foil was not heated through the chemical reaction. Instead, a focused laser beam was used to achieve this purpose. Through this process it was seen that the appearance of the folds in the foil was a direct result of the increase of the foil’s temperature. In order to more fully investigate these mechanical oscillations intensive mathematical simulations were done by two research groups at Princeton University (I. G. Kevrekidis, P. Holmes, J. E. Cisternas). The results of these calculations showed that the oscillations could only appear for a certain restricted set of parameters. For a fixed ratio between the thickness of the foil and its diameter only a small range of reaction parameters like temperature and partial pressure of the gases would produce the observed effects. In other words, the experimenters just had a stroke of luck!
The Princeton modeling revealed a delicate interplay between thermo-chemistry and thermo-mechanics. Transitions from strongly buckled to smooth states of the foil are mainly due to the oscillations of the chemical reaction itself: the creation of large amounts of carbon dioxide leads to the heating and folding of the foil. If the temperature of the catalyst rises, however, more of the carbon monoxide becomes oxidized until it is fully consumed in the immediate surrounding region. Thereafter the production of the carbon dioxide falls off leading to the contraction of the foil. Because of the slow rate of reaction, the lack of carbon monoxide can be replenished by the arrival of additional gas. Therefore, the entire process begins anew.
At the California Institute of Technology (F. Cirak, M. Ortiz) and at Rutgers University (A. M. Cuitiño ) further sophisticated computer calculations on the elastic deformations of an ultra-thin foil were undertaken. The resulting pictures were quite realistic. The fact that the foil folds very irregularly (first occurring when the plate holding the foil experiences a temperature difference of only one degree) is caused by the way in which it was manufactured. The thin foil is stretched over an approximately four-millimeter hole in a platinum holder and attaches itself to the platinum through adhesion alone. Although the foil over the hole is self-supporting, it is not stretched in a uniform manner. This tension works, at first, to stabilize the smooth form of the foil, so that with small temperature increases no folds appear. The computer simulations showed that if the samples were perfectly symmetrical, they would begin to bend with the smallest temperature increases. If the temperature continued to rise the bending would become more extreme, leading finally to a round hump in the sample.
Chemical-mechanical oscillations, although not including heat-coupling through chemical reactions, were first described 130 years ago in the first years of non-linear dynamics by Gabriel Lippmann in the “Annalen der Physik (Annals of Physics).” Then, for the first time, an iron nail in an aqueous oxidizing solution was brought into contact with a drop of mercury resting in a Petri dish, whereupon the mercury drop would begin to pulsate. Because of the resulting heart-shape of the oscillations, the phenomenon came to be called the “Quicksilver Heart.”
The researchers at the Fritz-Haber-Institute have already shown that the mechanical deformation of the catalytic foil can, in principle, also be used for direct measurement of the energy released by a chemical reaction. With a laser they heated a platinum foil just enough that, although no folds appeared, the foil immediately deformed when only one layer of carbon dioxide or oxygen particles reacted with their respective partner. These deformations can be combined with simple optical methods in order to develop ultra-sensitive instruments for the measurement of rates of chemical reactions.
Original Publication:
Fehmi Cirak, Jaime E. Cisternas, Alberto M. Cuitiño, Gerhard Ertl, Philip Holmes, Ioannis G. Kevrekidis, Michael Ortiz, Harm Hinrich Rotermund, Michael Schunack, Janpeter Wolff.
Oscillatory Thermo-Mechanical Instability of an Ultrathin Catalyst Science, 300, 1932-1935, 2003 Published 20th of June
You can find further information here:
Prof. Dr. Harm Hinrich Rotermund
Fritz-Haber-Institut der Max-Planck-Gesellschaft
Faradayweg 4-6, 14195 Berlin (Dahlem) Germany
Tel.: 049-030 – 8413–5129
Fax: 049-030 – 8413-5106
E-Mail: rotermun@fhi-berlin.mpg.de
Media Contact
More Information:
http://w3.rz-berlin.mpg.de/~rotermunAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















