Imaging study of key viral structure shows how HIV drugs work at atomic level
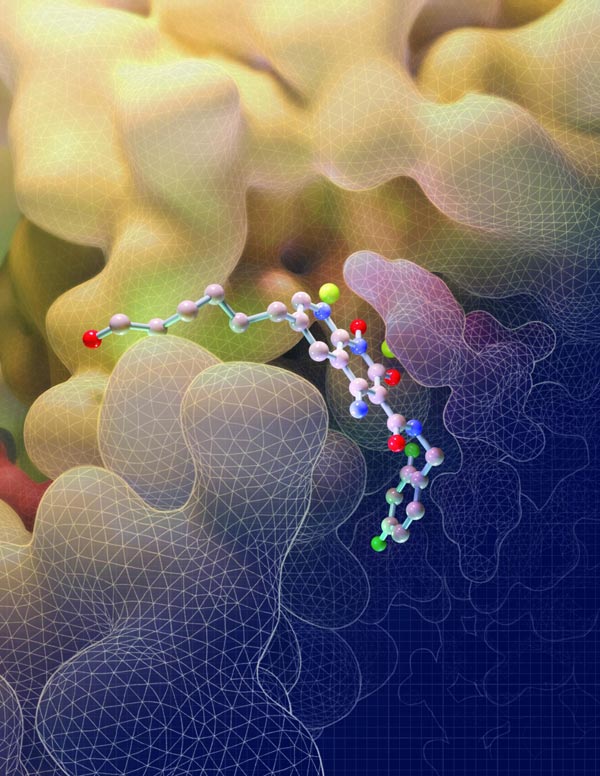
This illustration depicts the molecular structure of an HIV drug known as an INSTI binding to key sites on the intasome (yellow), the viral machine that allows HIV to invade cells. Credit: Salk Institute
Salk scientists have discovered how a powerful class of HIV drugs binds to a key piece of HIV machinery. By solving, for the first time, three-dimensional structures of this complex while different drugs were attached, the researchers showed what makes the therapy so potent. The work, which appeared in Science on January 30, 2020, provides insights that could help design or improve new treatments for HIV.
“The drugs we studied are the latest compounds available in the clinic today, as well as several important pre-clinical molecules. Until now, no one knew exactly how they bound to this HIV complex,” says the study's senior author Dmitry Lyumkis, an assistant professor in Salk's Laboratory of Genetics. “A better understanding of how the drugs work will help us improve them and design new therapeutic compounds.”
The intasome is a crucial structure of the virus that enables infection, composed of the HIV protein integrase and strands of viral DNA that form when the virus enters human cells. The intasome moves into each human cell and then carries out the chemical reactions necessary to integrate the virus' genetic material into human DNA.
Some drugs, called integrase strand transfer inhibitors (INSTIs), have managed to block the intasome; HIV can't infect human cells when the complex can't integrate viral DNA into the human genome. There are currently four INSTIs approved by the US Food and Drug Administration, as well as others under development.
Despite the success of these molecules, researchers have struggled to study how they inhibit the HIV intasome, largely due to difficulty in isolating intasomes for structural studies. In the past, most research on the intasome and INSTIs was carried out on another retrovirus called prototype foamy virus, or PFV. In 2017, Lyumkis and his colleagues were the first to determine the structure of purified HIV intasomes.
In the new work, Lyumkis' team went a step further: they obtained the structure of HIV intasomes while they were being actively blocked by one of four INSTIs–the commercially available drug bictegravir or three experimental compounds known as 4f, 4d and 4c. The team used tilted single-particle cryo-electron microscopy (cryo-EM), an imaging technique they've helped optimize, to reveal the structure of each intasome-drug complex.
The first observation that Lyumkis made was just how differently the drugs attached to the HIV intasome than what had been seen with the PFV intasome. The compound known as 4f, for instance, loops backwards onto itself as it binds to the PFV intasome but remains relatively flat as it attaches to the HIV version of the complex, details which can help researchers improve the binding properties of potential future molecules.
“To this day, everyone is still using the PFV intasome structure to rationalize and understand the mechanism of action of these drugs,” says Dario Passos, the study's co-first author and a staff scientist in Lyumkis' laboratory. “But we've shown that the field really needs to move and study the HIV structure if we want to make further progress.”
“We and many others have been working towards this goal for several decades and it is exciting that at long last we can now understand how HIV inhibitors work in detail and aid the development of new drugs.” says Min Li, co-first author and a staff scientist at the National Institute of Diabetes and Digestive and Kidney Diseases.
The structures also revealed why the drugs are so potent and what makes them so good at avoiding drug resistance. The INSTIs, Lyumkis and his colleagues found, fill the entire space that's normally occupied by DNA. That means if the HIV intasome develops a mutation that blocks the INSTI drugs from binding, it also blocks the DNA from attaching, rendering the complex useless for invading human cells.
Finally, the extremely high resolution of the structure obtained by the Salk researchers lets them see details on how the drugs chemically interacted with this binding pocket, and how INSTIs displaced water molecules to do so, which gave the team even more information on what makes INSTIs so successful in the clinic.
“In previous structures, we learned about intasome biology,” says Lyumkis. “But here, we've really started to gain insight into the therapeutic angle of how drugs can target these important viral assemblies.”
The researchers are planning additional work on the experimental drugs–focusing on the compound known as 4d, which, based on both preclinical testing and the new structural insight, shows more promise against HIV than other compounds. They also want to better understand what happens to the structure of the intasome in cases where it develops resistance to INSTIs. This could help them design more efficient drugs in the future, says Lyumkis.
###
Other researchers on the study were Ilona Jozwik and Youngmin Jeon of Salk; Renbin Yang and Robert Craigie of the National Institute of Diabetes and Digestive Diseases; Xue Zhi Zhao, Steven Smith, Stephen Hughes and Terrence Burke Jr. of the National Cancer Institute; and Diogo Santos-Martins and Stefano Forli of The Scripps Research Institute.
The work and the researchers involved were supported by grants from the National Institutes of Health, the Intramural Programs of the National Institute of Diabetes and Digestive Diseases, the Intramural Programs of the National Cancer Institute, and the Intramural AIDS Targeted Anti-Viral Program of the National Institutes of Health.
About the Salk Institute for Biological Studies: Every cure has a starting point. The Salk Institute embodies Jonas Salk's mission to dare to make dreams into reality. Its internationally renowned and award-winning scientists explore the very foundations of life, seeking new understandings in neuroscience, genetics, immunology, plant biology and more. The Institute is an independent nonprofit organization and architectural landmark: small by choice, intimate by nature and fearless in the face of any challenge. Be it cancer or Alzheimer's, aging or diabetes, Salk is where cures begin. Learn more at: salk.edu.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















