How viruses outsmart their host cells
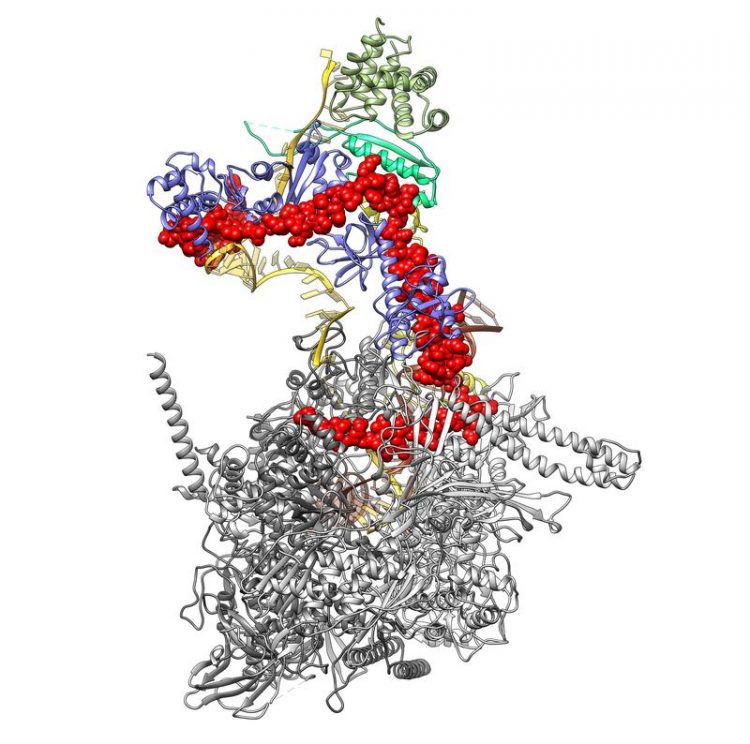
3D structure of the complex consisting of the bacterial RNA polymerase (light and dark gray) and the viral λN protein (red). The lower end of the λN reaches inside the RNA polymerase, linking its two halves. At its upper end, the λN protein is in contact with RNA (orange) and various regulatory proteins (yellow, blue and green). Credit: © Krupp/Charité
Viruses depend on host cells for replication, but how does a virus induce its host to transcribe its own genetic information alongside that of the virus, thus producing daughter viruses? For decades, researchers have been studying a type of bacteriophage known as 'lambda' to try and find an answer to this question.
Using high-resolution cryo-electron microscopy, a research group from Charité – Universitätsmedizin Berlin has now successfully deciphered this process. Their findings have been published in Molecular Cell*.
No host, no viruses. While it is true that viruses are capable of spreading by surviving outside a host, they need a host for replication. Viruses lack the complex apparatus necessary for the transcription of genetic information and its subsequent translation into new virus components.
This is why all viruses need access to a host cell's molecular infrastructure. For decades, researchers have been studying the ways in which viruses successfully exploit host functions. Their efforts have been focused on 'bacteriophages' – viruses that rely on bacterial hosts for replication. One of the most intensively studied and best characterized of these is the 'lambda phage'.
Previous research had shown that the lambda phage introduced its own genetic information into that of its host, inserting it at a specific site in the host genome. 'RNA polymerase', a protein complex responsible for transcribing genetic information, would normally stop reading this information at the end of the bacterial gene and would ignore any viral genes inserted behind it.
The virus uses a trick that prevents the RNA polymerase from terminating the transcription process: it introduces 'lambda-N' (λN), a tiny protein which attaches itself to the host's RNA polymerase and forces it to continue transcription of the viral genes.
Until now, and despite intensive efforts, researchers had failed to identify how this tiny protein can achieve such a feat. A Berlin-based team of researchers has now been able to visualize the 3D structure of the RNA polymerase-λN-complex using high-resolution imaging, enabling them to provide a detailed explanation of this 'viral exploitation'.
For their study, researchers from Charité worked with colleagues from Freie Universität Berlin and the Max Planck Institute for Molecular Genetics. They started by producing the individual components of this large protein complex separately. After reassembling the components, they placed the resulting complex in a thin film of water and froze it.
Using cryo-electron microscopy, the researchers took a total of 700,000 images of the protein complex from various angles, using these to compute its 3D structure. “The nature of this structure told us that the small viral λN protein seals together the two halves of the RNA polymerase, thus preventing it from falling apart once it reaches the stop signal at the end of the bacterial gene,” explains one of the study's first authors, Ferdinand Krupp, who is a doctoral student at Charité's Institute of Medical Physics and Biophysics.
“Because of this, the RNA polymerase continues transcribing even once it reaches the viral genes. Once all the viral genes have been read, they are then used as a blueprint for making daughter viruses – meaning the virus has achieved its objective,” says the biophysicist. He adds: “Our data also explain many of the individual results recorded over five decades of research. Taken together, these findings may contribute to the development of new antibacterial drugs.”
###
*Krupp F et al., Structural Basis for the Action of an All-Purpose Transcription Anti-Termination Factor. Mol Cell. 2019 Feb 19. doi: 10.1016/j.molcel.2019.01.016
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















