How cells stick together tightly
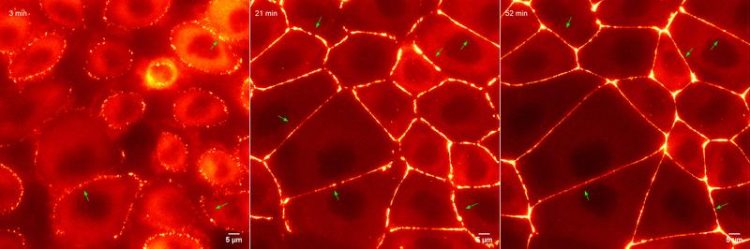
Formation of the tissue barrier: One of the proteins required to make the tight junction was labeled with a fluorescent dye. Using fluorescence microscopy, the labeled proteins were observed live. Beutel et al.
Our organs are specialized compartments, each with its own milieu and function. To seal our organs, the cells in the tissue must form a barrier which is tight even down to the level of molecules. This barrier is formed by a protein complex that “sticks” all the cells together without any gaps.
The loss of this barrier can lead to many diseases, including the infiltration of pathogens into our internal system. Since maintaining the tissue barrier is central to our organ functions, it is important to understand how this barrier is created between cells.
Researchers at the Max Planck Institute for Molecular Cell Biology and Genetics (MPI-CBG) in Dresden discovered that cells use a process similar to condensation of water droplets on a cold window to form cell junctions.
Specific proteins condense as droplets on the cell membrane when neighboring cells touch. These droplets enrich all the components required to create a stable barrier between cells. The results are published in the journal Cell.
The surface of our organs is lined with a layer of epithelial cells. These cells play an important role in protecting our organs, adapting them to a changing environment, and transporting molecules in and out of tissues. What seals epithelial tissue are tight junctions that act as the molecular barrier between cells.
A key question for scientists was how this molecular complex robustly assembles between cells. The team around MPI-CBG research group leader Alf Honigmann set out to piece together the building blocks that could explain how tight junctions are built.
One key building block of tight junctions is the family of ZO proteins which are located on the inside of cells. Using a combination of tissue imaging, gene editing, and biochemistry, the researchers found that the proteins inherently contain regions that allow them to stick to one another and form droplets on the membrane of epithelial cells. This ability allows the ZO proteins to become more concentrated and to selectively sequester adhesion proteins, needed for tight junction assembly.
Oliver Beutel, the first author of the study, explains: “This process is somewhat similar to the phenomenon of water condensing on a cold glass window, spontaneously forming liquid droplets and streams. In the case of tight junctions, the ZO proteins condense on the inside of epithelial cell membranes to form a hub where all the necessary components can be encapsulated to assemble into a tight junction.” These findings suggest that the properties of ZO proteins can serve as a template to reconstruct the architecture and function of tight junctions.
Alf Honigmann, the supervisor of the study, concludes: “This study reveals an important mechanism, which epithelial cells use to establish tight junctions. Our work on this protein machinery exemplifies the principles how cells leverage simple physical phenomena like condensation to assemble complex molecular structures.” He adds: “Our findings imply exciting possibilities how junctions can quickly remodel during tissue growth and repair.”
Dr. Alf Honigmann
+49 (0) 351 210 2969
honigmann@mpi-cbg.de
Oliver Beutel, Riccardo Maraspini, Karina Pombo-García, Cécilie Martin-Lemaitre, Alf Honigmann: “Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions” Cell, 31. October, 2019, doi: https://doi.org/10.1016/j.cell.2019.10.011
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















