New muscular disease: Myoglobinopathy
Institute (IDIBELL) led by Dr. Montse Olivé have described in Nature Communications a new muscular disease caused by a mutation in the myoglobin gene. The study has been possible thanks to a collaboration with a group of geneticists from the University of Western Australia (UWA), led by Prof. Nigel Laing, and researchers from the Karolinska Institute (Stockholm, Sweden). Credit: Bellvitge Biomedical Research Institute (IDIBELL)
Researchers of the Bellvitge Biomedical Research Institute (IDIBELL) led by Dr. Montse Olivé have described in Nature Communications a new muscular disease caused by a mutation in the myoglobin gene.
The study has been possible thanks to a collaboration with a group of geneticists from the University of Western Australia (UWA), led by Prof. Nigel Laing, and researchers from the Karolinska Institute (Stockholm, Sweden).
Myoglobin, the protein that gives muscles their red colour, has as its main function the transportation and intracellular storage of oxygen, acting as an oxygen reservoir when there are low levels (hypoxia) or a total lack thereof (anoxia). It also acts as scavenger of free radicals and other reactive oxygen species, avoiding cell damage due to oxidative stress.
“This is the first time that a disease caused by a mutation in the myoglobin gene has been identified”, says Montse Olivé, doctor at the Bellvitge University Hospital, responsible for the muscular disease area and researcher of the Human Molecular Genetics group of the Genes, Disease and Therapy at IDIBELL.
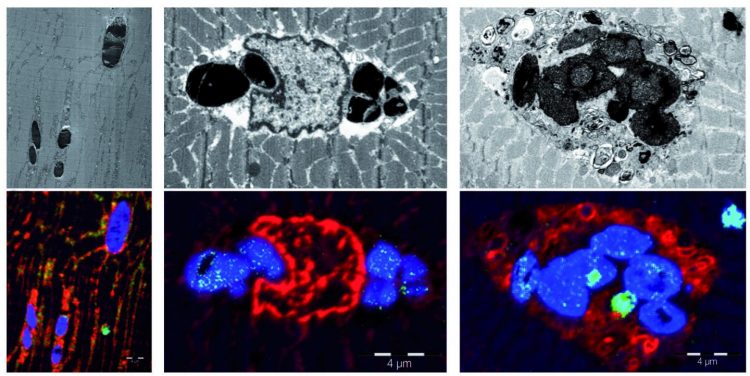
“We have identified the same mutation in several members of 6 unrelated European families, all of whom had the same symptoms and showed very characteristic lesions – called sarcoplasmic bodies – in muscle biopsies,” adds Dr Olivé.
Myoglobinopathy, the name that has been proposed for the new disease, manifests between the fourth and fifth decade of life. It causes a progressive weakness of the axial and limb muscles, and in more advanced stages affects the respiratory musculature and the heart.
The study has also involved, among others, researchers from the ALBA synchrotron and the Autonomous University of Barcelona (UAB). The infrared microscopy of the MIRAS beamline at the synchrotron, which is able to characterize the chemical composition and structure of proteins, allowed researchers to demonstrate the presence of oxidized lipids in the damaged muscle cells. “We have observed that sarcoplasmic bodies correspond to oxidized lipids and misfolded proteins”, says Nuria Benseny, a postdoctoral researcher at the ALBA synchrotron.
Finally, researchers from the universities of Modena and Vienna have identified an alteration in the biochemical and thermodynamic properties of the mutated myoglobin, including an alteration in the oxygen-binding capacity that contributes to the formation of the lipid and protein aggregates that are found in the muscles of the patients.
This discovery has allowed to establish the diagnosis for some patients after many years and will allow researchers to focus on finding a cure for this disease from now on.
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















