Optical tweezers unveil a secret of muscle power: What holds the heart together
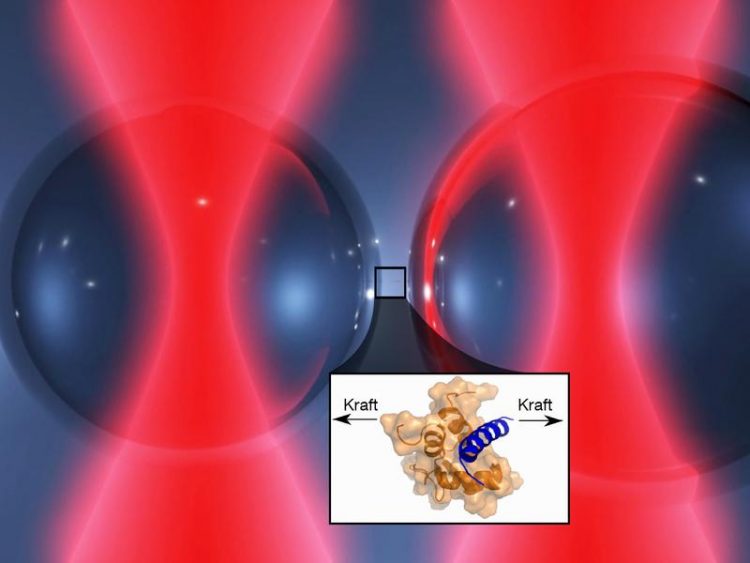
Optical tweezers: Two laser beams hold tiny glass beads. The protein complex ist fixed on the surface. If the glass beads are moved apart, the protein molecules are stretched Image: Marco Grison / TUM
The human body is a never-ending construction site: Proteins are permanently being decomposed and replaced. But this perpetual reconstruction does not inhibit the body’s functionality. The heart keeps beating, respiration does not halt, we can rely on our eyes and ears around the clock.
How the body manages to keep together the protein strands in muscles even while individual building blocks are replaced has preoccupied Prof. Matthias Rief for many years. “There must be forces that stabilize the individual chains, the filaments. Otherwise, muscles would fall apart. But, until now, nobody has tracked down the source of these forces,” reports the chair of the Department of Biophysics at TU Munich. In collaboration with his team, he has now deciphered the secret behind the cohesion of muscles.
Optical tweezers decipher the bonding forces
As it turns out, two proteins are responsible for allowing muscles to expand without falling apart. The first is titin, the longest protein in the human body. The second is α-actinin, which anchors titin to the muscle tissue.
The TUM researchers studied the interactions between these proteins using a specially developed apparatus: The “optical tweezers” fill a 20-square meter room in the basement of the institute. There are laser sources, optics, cameras and monitors. The heart of the facility is a measuring chamber filled with a fluid and small globules of glass. The titin and α-actinin molecules stick to the surface of these globules. Pairs of laser beams that penetrate the measuring cell catch one globule each to hold them fast.
A network gives strength
“Using the laser beams we can position the globules close enough to each other that the two proteins can interlink,” explains Marco Grison, who is researching the bonding between the muscle building blocks in the context of his doctoral dissertation. “In a second step, we increase the distance between the laser beams and, thereby, between the globules, until the proteins are stretched to their limit. The bonding force between titin and α-actinin can then be calculated from this distance.”
The protein bond can resist a force of five piconewtons – which corresponds roughly to one billionth the weight of a bar of chocolate. “This result left us very surprised,” recalls Rief. “Such small forces should not be able to hold a muscle together for long.”
And yet, the protein link is the key to understanding: In muscles, each titin strand is held by up to seven α-actinin proteins. This was determined by the structural biologists at the University of Vienna, with whom the Munich researchers are collaborating. This increases the force seven-fold. Enough to allow the heart to beat and even decompose and replace individual molecular chains – right alongside normal operation, so to speak.
Repairs during normal operation
“In total, the bonds are sufficient to stabilize the muscle,” explains Rief. “The protein network is not only stable, but also extremely dynamic. Our measurements show that the proteins release their bond when they are pulled apart. But, as soon as the stretching force abates they reconnect.” This affinity of protein molecules for one another ensures that muscles do not tear, but rather revert to their original form after being stretched.
This tight bond between the proteins is not limited to the heart. The interaction between titin and α-actinin stabilizes all muscles when they are stretched – regardless of whether during breathing, walking, grasping or laughing.
Basic research for future medicine
Patients may one day profit from these results: “The basic research provides the basis for understanding genetic ailments like muscular dystrophy and congestive heart failure,” sums up Rief. “That could help doctors and pharmacologists develop new therapies.”
The research was funded by the EU (Marie Curie Initial Training Network), the German Reseach Foundation (FOR 1352, Clusters of Excellence Nanosystems Initiative Munich, NIM and Center for Integrated Protein Science Munich, CIPSM), the Austrian Science Fund and the Austrian Ministry of Science, Research and Economics (Center for Optimized Structural Studies, “Laura Bassi Centres of Expertise” program).
Publication:
α-Actinin/titin interaction: A dynamic and mechanically stable cluster of bonds in the muscle Z-disk,
Marco Grison, Ulrich Merkel Julius Kostan, Kristina Djinovic-Carugo, and Matthias Rief
http://www.pnas.org/content/early/2017/01/11/1612681114.abstract
Contact:
Prof. Dr. Matthias Rief
Technical University of Munich
Chair of Molecular Biophysics (E22)
James-Franck-Str.1, 85748 Garching, Germany
Tel.: +49 89 289 12471 – E-Mail: matthias.rief@mytum.de
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















