Breaking a chemical bond
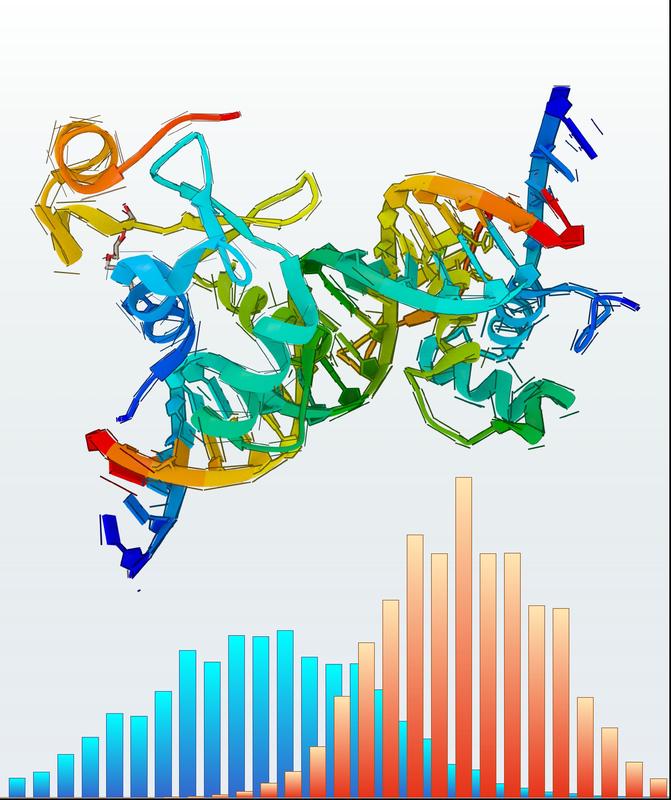
The illustration schematically depicts a protein structure and rupture force distributions of one of its macromolecular bonds under load for two different loading rates. Graph: Leipzig University, Institute of Theoretical Physics
The fundamental question how a molecular bond breaks is of interest in many fields of science and has been studied extensively. Yet, now writing in Nature Communications, a group of theoretical physicists from the University of Leipzig, Germany, has put forward a more powerful analytical formula for forcible bond breaking than previously available.
It predicts how likely a bond will break at a given load, if probed with a prescribed loading protocol. This so-called rupture force distribution is the most informative and most commonly measured quantity in modern single-molecule force spectroscopy experiments (which may roughly be thought of as nanoscopic versions of the conventional crash- or breaking tests employed in materials science and engineering).
Such experiments are nowadays performed in large numbers in molecular biology and biophysics labs to probe the mechanical strength of individual macromolecular bonds.
Recent methodological advances have pushed force spectroscopy assays to ever higher loading rates (the equivalent of the speed employed in the macroscopic crash-test). This provided a strong incentive for the Leipzig team to improve on current state-of-the-art theories for forcible bond breaking, which are limited to comparatively low speeds.
Moreover, the new equation solves another problem that has bothered experts in the field for many years. Force spectroscopy experiments are often simulated with sophisticated all-atom computer models to supplement the experimental data with information on internal molecular details that cannot be resolved in a laboratory setting.
However, because of their enormous complexity, such computer simulations operate at extremely high loading rates to cut down on the runtime. As a consequence, simulation and experiment were so far two essentially distinct branches of force spectroscopy.
The new equation, which gives exact results for both low and high loading rates, will thus suit both experimentalists and computer scientists, and help them to systematically analyze and compare their results.
This should eventually improve our microscopic understanding of the strength of synthetic materials and of how proteins attain and maintain their three-dimensional structure and perform conformational changes, which are core features determining the function and dysfunction of these amazing engines of life.
Article in „Nature Communications”:
„Theory of rapid force spectroscopy“,
by Jakob T. Bullerjahn, Sebastian Sturm and Klaus Kroy
doi:10.1038/ncomms5463
Contact:
Prof. Dr. Klaus Kroy
Phone: +49 341 97 32436
E-Mail: klaus.kroy@uni-leipzig.de
http://www.nature.com/ncomms/2014/140731/ncomms5463/full/ncomms5463.html
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















