Study shows how water dissolves stone, molecule by molecule
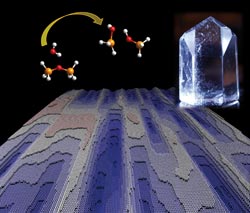
The dissolution process of a crystalline structure in water is shown: two bonded SiO4 -- molecules dissolve (top left), a quartz crystal (top right) and the computer-simulated surface of a dissolving crystalline structure (below). <br><br>CREDIT: MARUM & Rice University<br>
Scientists from Rice University and the University of Bremen's Center for Marine Environmental Sciences (MARUM) in Germany have combined cutting-edge experimental techniques and computer simulations to find a new way of predicting how water dissolves crystalline structures like those found in natural stone and cement.
In a new study featured on the cover of the Nov. 28 issue of the Journal of Physical Chemistry C, the team found their method was more efficient at predicting the dissolution rates of crystalline structures in water than previous methods. The research could have wide-ranging impacts in diverse areas, including water quality and planning, environmental sustainability, corrosion resistance and cement construction.
“We need to gain a better understanding of dissolution mechanisms to better predict the fate of certain materials, both in nature and in man-made systems,” said lead investigator Andreas Lüttge, a professor of mineralogy at MARUM and professor emeritus and research professor in Earth science at Rice. His team specializes in studying the thin boundary layer that forms between minerals and fluids.
Boundary layers are ubiquitous in nature; they occur when raindrops fall on stone, water seeps through soil and the ocean meets the sea floor. Scientists and engineers have long been interested in accurately explaining how crystalline materials, including many minerals and stones, interact with and are dissolved by water. Calculations about the rate of these dissolution processes are critical in many fields of science and engineering.
In the new study, Lüttge and lead author Inna Kurganskaya, a research associate in Earth science at Rice, studied dissolution processes using quartz, one of the most common minerals found in nature. Quartz, or silicon dioxide, is a type of silicate, the most abundant group of minerals in Earth's crust.
At the boundary layer where quartz and water meet, multiple chemical reactions occur. Some of these happen simultaneously and others take place in succession. In the new study, the researchers sought to create a computerized model that could accurately simulate the complex chemistry at the boundary layer.
“The new model simulates the dissolution kinetics at the boundary layer with greater precision than earlier stochastic models operating at the same scale,” Kurganskaya said. “Existing simulations rely on rate constants assigned to a wide range of possible reactions, and as a result, the total material flux from the surface have an inherent variance range — a plus or minus factor that is always there.”
One reason the team's simulations more accurately represent real processes is that its models incorporate actual measurements from cutting-edge instruments and from high-tech materials, including glass ceramics and nanomaterials. With a special imaging technique called “vertical scanning interferometry,” which the group at MARUM and Rice helped to develop, the team scanned the crystal surfaces of both minerals and manufactured materials to generate topographic maps with a resolution of a just a few nanometers, or billionths of a meter.
“We found that dissolution rates that were predicted using rate constants were sometimes off by as much as two orders of magnitude,” Lüttge said.
The new method for more precisely predicting dissolution processes could revolutionize the way engineers and scientists make many calculations related to a myriad of things, including the stability of building materials, the longevity of materials used for radioactive waste storage and more, he said.
“Further work is needed to prove the broad utility of the method,” he said. “In the next phase of research, we plan to test our simulations on larger systems and over longer periods.”
The research was supported by the Global Climate and Energy Project at Stanford University.
VIDEO is available at:
http://youtu.be/cfgPVxWJaB4
CREDIT: R. Arvidson/MARUM
A copy of the Journal of Physical Chemistry C paper is available at: http://pubs.acs.org/doi/abs/10.1021/jp408845m
This release can be found online at news.rice.edu.
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation's top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,708 undergraduates and 2,374 graduate students, Rice's undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice has been ranked No. 1 for best quality of life multiple times by the Princeton Review and No. 2 for “best value” among private universities by Kiplinger's Personal Finance. To read “What they're saying about Rice,” go to http://tinyurl.com/AboutRiceU.
Media Contact
More Information:
http://www.rice.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















