Now researchers can see how unfolded proteins move in the cell
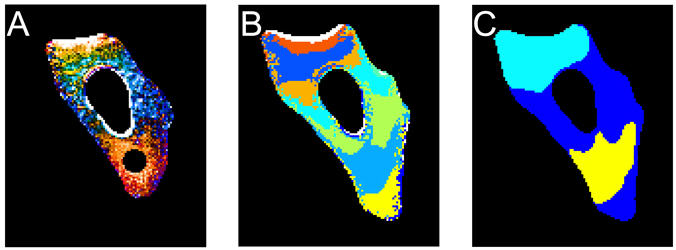
By looking at the dynamics of how the unfolded protein moved in the cell (A), the researchers mapped areas in the cell with different rates of diffusion (B and C). | Photo courtesy Hannah Gelman
Studying the relationship between protein folding and transport could provide great insight into protein-misfolding diseases such as Alzheimer’s and Huntington’s. Chemistry professor Martin Gruebele and graduate students Minghao Guo and Hannah Gelman published their findings in the journal PLOS ONE.
“We’re looking at the earliest stages of disease, the initial phases of transport of bad proteins,” Gruebele said. In the past, he said, much research on Alzheimer’s and similar disease focused on fibrils, large bundles of misfolded proteins that form in the brain.
“But now, we think the fibrils are just an end product that’s left over when the cell dies, and the actual killing mechanism has to do with migration of the protein to specific places in the cell like the outer membrane,” he said. “Understanding how these mechanisms work at a fundamental level is going to give people more handles on where to look to cure things.”
Researchers have hypothesized that an unfolded protein moves more slowly through the cell, because it would be a big, stringy mess rather than a tightly wrapped package. The Illinois team devised a way to measure how diffusion slows down when a protein unfolds using a fluorescence microscope, then used three-dimensional diffusion models to connect the protein’s unfolding to its motion.
The researchers found that the unfolded protein did indeed slow down, although its speed was not steady. It sometimes zoomed swiftly to a new location, and sometimes sat idling in one area, like a vehicle in stop-and-go rush-hour traffic. They were able to map out areas of the cell with different rates of diffusion, the cellular version of a speed limit.
The unfolded protein’s slowdown is not only due to size, however. The researchers did additional experiments to prove that the unfolded protein stuck to other molecules in the cell. A class of molecules in the cell called chaperones have the job of binding to parts of proteins that come unfolded, and the researchers found that the unfolded protein interacted more with chaperones than did the properly folded protein. However, when high numbers of proteins unfold, the cell’s systems can get overloaded and the chaperones can’t handle them all.
“Looking at something like this can start to give people a handle on why something that seems relatively harmless in vitro sometimes can have such a large effect in the cell,” Gelman said. “A change that makes a slightly less effective protein in the test tube can turn into a completely fatal mutation in the cell. First, the protein’s role in the cell can no longer be fulfilled. Second, as more and more unfold, they can disrupt the function of the whole cell.”
The researchers think that the unfolded protein is likely to stick to nonchaperone molecules, as well, causing other problems in the cell and disrupting the flow within a cell. They plan to use the specialized microscope to study other proteins and how unfolding affects their diffusion, to see if the properties they observed are universal or if each protein has its own response.
They also hope to use their method to watch how unfolded or misfolded proteins move to the cell’s membrane, where they aggregate and create the problems seen in Alzheimer’s and other diseases.
“There’s a whole cascade of things,” Gruebele said. “If you have a single car accident in the middle of nowhere, it’s really only a problem for the owner. But if you have a single car that stops in the middle of the road on the freeway in Los Angeles, very soon the entire freeway is going to be backed up. What we’re looking at is like the car stopping on the freeway. We’re not worried yet about what happens to the line of cars an hour later – that’s the fibril.”
The National Science Foundation supported this work.
Editor's note: To reach Martin Gruebele, call 217-333-1624; email: gruebele@scs.illinois.edu.
The paper, “Coupled Protein Diffusion and Folding in the Cell,” is available online.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















