Breaking through a double wall with a sledgehammer
Formation of new nuclear pore complexes with and without Nup153 Benjamin Vollmer/Friedrich Miescher Laboratory
The nucleus is the control center of the cell. Well protected by a double membrane, the DNA, our genetic material, is located within the nucleus. But it is not an impervious compartment. There is a lively, but well controlled exchange of proteins and other molecules between the nucleus and the cytoplasm.
In order to control the exchange, there are so called nuclear pore complexes located within the nuclear membrane. In a typical human cell there are around 3000 of them, each performing an astonishing number of about 1000 transport events per second – similar to a motorway in the cell.
Nuclear pore complexes belong to the biggest protein complexes of the cell but are only composed of around 30 different proteins, the so-called nucleoproteins or Nups. Smaller molecules, such as water, cross the membrane by diffusion. Proteins are much bigger and have to be actively transported in an energy dependent process. The import is conducted by transport receptors that recognize and bind the protein to be transported, the so-called cargo.
In the nucleus they bind to another molecule, the GTPase Ran, that is responsible for transport receptor recycling. When Ran binds transport receptors, the cargo is set free and can fulfill its function in the nucleus. The export is also performed by transport receptors that bind the cargo in the nucleus and carry it out into the cytoplasm.
How transport through the nuclear pore works is largely understood. However, it is unclear how the nuclear pore complex assembles from the different nucleoporins. Benjamin Vollmer and Michael Lorenz, PhD students in Wolfram Antonin’s research group Nuclear Envelope Dynamics, recently shed light on this issue. They analyzed the behavior of Nup153, one of the nucleoporins.
The scientists could show that Nup153 not only plays a role in the already existing nuclear pore complexes, but that it is also crucial for the insertion of new pores into the nuclear envelope. Nup153 binds the so-called Y-complex, an important structural element of the nuclear pore complexes. It also contains binding sites for various transport receptors and for the GTPase Ran. It is therefore likely that Nup153 is important for the import of cargo to the nucleus.
Antonin and his group investigated whether Nup153 was important for the formation of the nuclear pore itself using biochemical approach. They removed the protein from a cell extract that contained all other components of the cell. In this way, researchers have been able to understand which constituents of the cell are important for a given process.
If DNA was added to this cell extract a new nuclear envelope formed around it, just as it does during cell division. In fact, an intact nuclear envelope and nuclear pore complexes also formed without Nup153. But at certain spots they aggregated, forming clusters of nuclear pore complexes.
In order to prove a theory, scientists often aim to refute the contrary. So their idea was the following: if Nup153 is added to the cell extract, a nuclear membrane should assemble with evenly distributed nuclear pore complexes, which was indeed the case. When they tried the same with a mutant of the protein that is not able to bind to the membrane they observed that the nuclei were smaller.
This could have two reasons. First, the nuclear import of proteins might not work correctly, since the nuclei only grow to their full size if enough new proteins enter the nucleus. However, the researchers could refute this. The other explanation could be that fewer nuclear pore complexes formed in the absence of Nup153 membrane binding.
Nuclear pore complexes do not only form during cell division upon reassembly of the nuclear membrane but also in the interphase between two cell divisions. The latter process is much less understood than pore formation during cell division. Here, the pore complex has to be inserted into an already existing double membrane.
“Imagine you want to have a window in a thick wall. If you haven’t inserted a window during the construction phase you have to use a sledgehammer first and break a hole into the wall”, says Wolfram Antonin. When Nup153 is inserted into the double membrane via its membrane binding domain tension is generated and the membrane inclines, allowing the pore to form.
To show that Nup153 initiates pore assembly in the intact nuclear envelope, Antonin and his coworkers counted nuclear pore complexes. If Nup153 was missing there were fewer pore complexes. This could be reverted by adding Nup153 – but not with the mutant not binding to the membrane. To characterize the function of Nup153, the scientists investigated the interactions with other proteins.
“Nup153 binds to Ran and the Y-complex. Therefore it seemed obvious that Nup153 helps to bring these proteins to the membrane”, says Benjamin Vollmer, one of the authors of the study.
To address the questions whether Nup153 recruits Ran or the Y-complex to the nuclear membrane, two artificial proteins were assembled. The membrane-binding domain of Nup153 was either fused to the Y-complex binding domain or the Ran binding domain. The fusion proteins were added to cell extract without Nup153, as in the experiment where the smaller nuclei had formed. Normal sized nuclei were observed in the presence of the protein containing the membrane binding and the Y-complex binding domains.
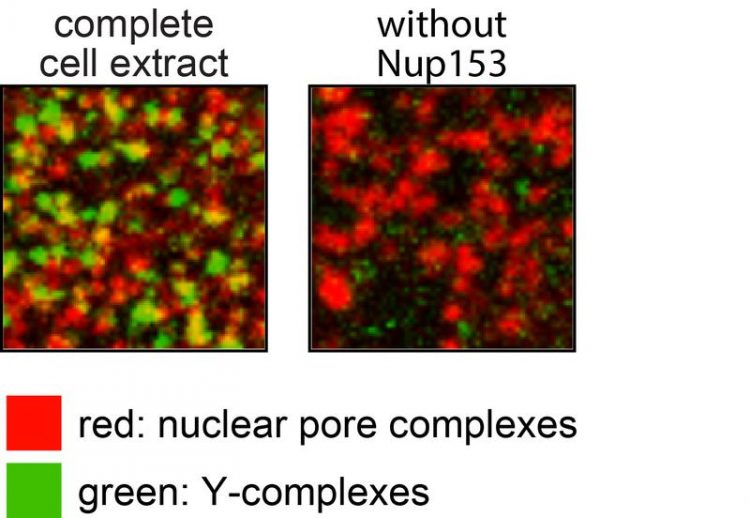
To confirm that Nup153 indeed recruits the Y-complex to newly forming pores in an already assembled nucleus, the scientists made the cells glow: the old and new nuclear pore complexes were stained with a red fluorescent dye. Additionally, the Y-complex was labeled with a green fluorescent protein.
Newly forming pore complexes therefore contained red and green color and could be differentiated from previously formed complexes. If Nup153 was removed from the experiment there was no more green glowing visible, indicating that without Nup153 the formation of new nuclear pore complexes during interphase was inhibited.
“Double membranes do not only exist around the nucleus”, says co-author Michael Lorenz, “and Nup153 is produced in the cytoplasm. We asked ourselves, why nuclear pore complexes don´t appear at other sites within the cell”. The key is the transport function of nuclear pore complexes itself. Nup153 has to be imported as any other protein by the transport receptor transportin.
The researchers at the FML have discovered that the binding site for transportin lies close to the membrane-binding site of Nup153. If the newly synthesized Nup153 is bound by transportin in the cytoplasm the membrane-binding site is blocked. When Nup153 is successfully imported to the nucleus transportin dissociates from its cargo Nup153. This dissociation requires the GTPase Ran, which is only present in abundance within the nucleus.
However, the cell can be tricked. If the researchers increased the concentration of Ran in the cytoplasm, nuclear pore complexes could also form at other sites in the cell. Such misplaced nuclear pore complexes can be found in some cancer cells. The role Nup153 in the healthy or sick organism remains to be seen. This requires that the whole process of the formation of the nuclear pore complex and its function are to a large extent understood.
Original Publication:
Vollmer et al.: Nup153 Recruits the Nup107-160 Complex to the Inner Nuclear Membrane for Interphasic Nuclear Pore Complex Assembly, Developmental Cell (2015)
DOI: http://dx.doi.org/10.1016/j.devcel.2015.04.02
Media Contact
More Information:
http://eb.mpg.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















