Argon is not the 'dope' for metallic hydrogen
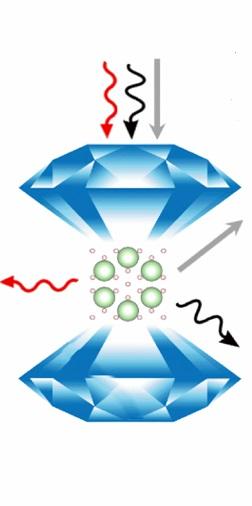
This is an illustration of Ar(H2)2 in the diamond anvil cell. The arrows represent different ways that spectroscopic tools study the effect of extreme pressures on the crystal structure and molecular structure of the compound. (For experts, the red arrow represents Raman spectroscopy, the black arrow represents synchrotron x-ray diffraction, and the gray arrow represents optical absorption spectroscopy.) Image courtesy of Cheng Ji
“Although theoretically ideal for energy transfer or storage, metallic hydrogen is extremely challenging to produce experimentally,” said Ho-kwang “Dave” Mao, who led a team of physicists in researching the effect of the noble gas argon on pressurized hydrogen.
It has long been proposed that introducing impurities into a sample of molecular hydrogen, H2, could help ease the transition to a metallic state. So Mao and his team set out to study the intermolecular interactions of hydrogen that's weakly-bound, or “doped,” with argon, Ar(H2)2, under extreme pressures. The idea is that the impurity could change the nature of the bonds between the hydrogen molecules, reducing the pressure necessary to induce the nonmetal-to-metal transition. Previous research had indicated that Ar(H2)2 might be a good candidate.
Surprisingly, they discovered that the addition of argon did not facilitate the molecular changes needed to initiate a metallic state in hydrogen. Their findings are published by the Proceedings of the National Academy of Sciences.
The team brought the argon-doped hydrogen up to 3.5 million times normal atmospheric pressure–or 358 gigapascals–inside a diamond anvil cell and observed its structural changes using advanced spectroscopic tools.
What they found was that hydrogen stayed in its molecular form even up to the highest pressures, indicating that argon is not the facilitator many had hoped it would be.
“Counter to predictions, the addition of argon did not create a kind of 'chemical pressure' on the hydrogen, pushing its molecules closer together. Rather, it had the opposite effect,” said lead author Cheng Ji.
###
The study's other co-authors are: Carnegie's Alexander Goncharov, Dmitry Popov, Bing Li, Junyue Wang, Yue Meng, Jesse Smith, and Wenge Yang; as well as Vivekanand Shukla, Naresh Jena, and Rajeev Ahuja of Uppsala University; and Vitali Prakpenka of the University of Chicago.
This work was supported by the U.S. National Science Foundation, the U.S. Department of Energy, the Chinese Academy of Sciences Visiting Professorship for Senior International Scientists and Recruitment of Foreign Experts, the European Erasmus Fellowship program, and the Swedish Research Council.
The Carnegie Institution for Science (carnegiescience.edu) is a private, nonprofit organization headquartered in Washington, D.C., with six research departments throughout the U.S. Since its founding in 1902, the Carnegie Institution has been a pioneering force in basic scientific research. Carnegie scientists are leaders in plant biology, developmental biology, astronomy, materials science, global ecology, and Earth and planetary science.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles
Faster, more energy-efficient way to manufacture an industrially important chemical
Zirconium combined with silicon nitride enhances the conversion of propane — present in natural gas — needed to create in-demand plastic, polypropylene. Polypropylene is a common type of plastic found…

Energy planning in Ghana as a role model for the world
Improving the resilience of energy systems in the Global South. What criteria should we use to better plan for resilient energy systems? How do socio-economic, technical and climate change related…

Artificial blood vessels could improve heart bypass outcomes
Artificial blood vessels could improve heart bypass outcomes. 3D-printed blood vessels, which closely mimic the properties of human veins, could transform the treatment of cardiovascular diseases. Strong, flexible, gel-like tubes…





















