Taking the temperature of the periodic system

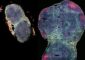
Resembling the spectrum of a light bulb at 9,000 K (8727 °C / 15740 °F), the distribution of all known spectral lines may yield insights into the early universe. Universität Rostock / Matthias Heinrich
Playfully browsing this extensive database, a German team of scientists from the University of Rostock was quite astonished to discover an entirely unexpected regularity:
Plotting the number of known lines over the wavelength, the distribution bears a striking resemblance to the familiar Planckian distribution of thermal radiation emitted by an incandescent light source.
With most of the lines occurring at 320 nm, just beyond what would be perceived as blue by the human eye, the temperature of this hypothetical light bulb would have to be 9,000 K (8727 °C / 15740 °F).
This seemingly random value may suggest an unexpected connection to cosmology: Shortly after the “big bang”, the early universe was dominated by pure energy.
As it subsequently expanded and cooled down, the elementary particles began to condense and form the matter that makes up the known universe today.
This fateful transition took place at 9,000 K. Pure coincidence? Perhaps. Nevertheless, the researchers nevertheless felt this discovery to be curious enough to turn to the broader scientific community to search for a more satisfying explanation.
After all, as was so aptly stated by the late great Isaac Asimov, “the most exciting phrase to hear in science, the one that heralds new discoveries, is not `Eureka!' (I found it!) but ‘That's funny…’ ”.
Prof. Dr. Alexander Szameit
AG Experimentelle Festkörperoptik
Institut für Physik
Universität Rostock
Tel.: +49 381 498-6790
E-Mail: alexander.szameit@uni-rostock.de
The original publication in “Annals of Physics” is available under https://doi.org/10.1002/andp.202000033.
Media Contact
More Information:
http://www.uni-rostock.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
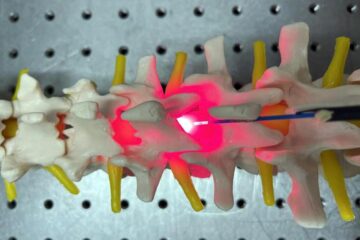
Red light therapy for repairing spinal cord injury passes milestone
Patients with spinal cord injury (SCI) could benefit from a future treatment to repair nerve connections using red and near-infrared light. The method, invented by scientists at the University of…
Insect research is revolutionized by technology
New technologies can revolutionise insect research and environmental monitoring. By using DNA, images, sounds and flight patterns analysed by AI, it’s possible to gain new insights into the world of…

X-ray satellite XMM-newton sees ‘space clover’ in a new light
Astronomers have discovered enormous circular radio features of unknown origin around some galaxies. Now, new observations of one dubbed the Cloverleaf suggest it was created by clashing groups of galaxies….