Smart Keys for cancer therapy
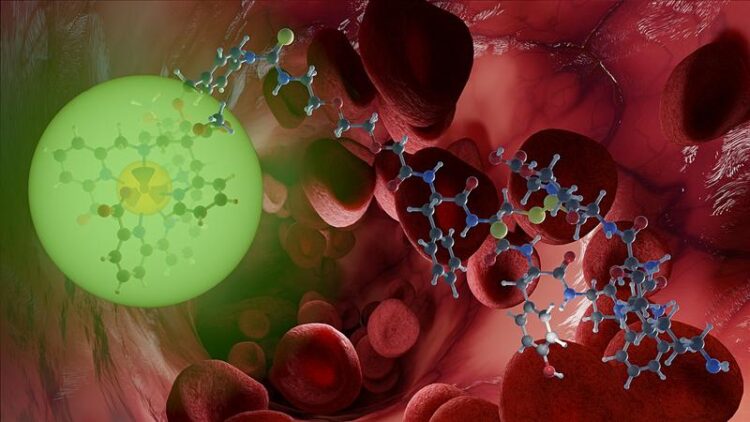
A suitable combination of radioactive elements in so-called radionuclide theranostics allows the visualization and treatment of cancer to be combined in a targeted manner.
Credit: B. Schröder/HZDR
Research team develops new system for imaging and treating tumors.
Thanks to the radiation they emit, radioactive compounds are suited both to imaging and treating cancers. By appropriately combining them in novel, so-called radionuclide theranostics, both applications can be dovetailed. A radiopharmacy team at Helmholtz-Zentrum Dresden-Rossendorf (HZDR) and Heidelberg University has now presented such a system in the Journal of the American Chemical Society (DOI: 10.1021/jacs.2c08438) that successfully solves one of the biggest problems to date: it works at physiologically relevant temperatures.
“Basically, we can think of it as functioning like a smart key that we use to control our automobiles. We use so-called radionuclides, i.e., unstable atomic nuclei, that spontaneously emit ionized radiation when they decay. We track down the tumor with a diagnostic radionuclide. The targeted internal irradiation close to the diseased tissue is then taken on by a different, therapeutic radionuclide,” says Dr. Manja Kubeil of HZDR’s Institute of Radiopharmaceutical Cancer Research, describing her theranostic approach.
Her team in the Department of Radionuclide Theragnostics develops exactly these types of substance to track and destroy tumors. Thus, the researchers employ matched pairs of radionuclides which, due to their decomposition characteristics, can be used both for imaging and for tumor therapy on the same target molecule.
The relevant radionuclide is stably bound in what is known as a chelator and linked to a biomolecule by a kind of chemical bridge. “The word chelator comes from the Latin; its stem relates to being encircled by the claws of a crayfish. We prefer the image of a molecular cage that firmly encloses the radionuclide so that it can’t spread in the body uncontrollably. The target-seeking biomolecule for its part must fit perfectly with the docking site on the cancer cells, just like a key in a lock. The radionuclide then accumulates on the tumor tissue and exclusively develops its destructive impact there – that’s the idea,” says Kubeil.
Stable bonds at practicable temperatures
Lutetium-177, for instance, is particularly suitable as a beta emitter for releasing electrons to treat various tumors as well as a source of gamma rays for imaging. Actinium-225, an alpha emitter that can be used for efficient treatment, is even more effective in destroying tumors and is also very tightly bound by the chelator. Neither radionuclide occurs naturally on Earth. Appropriate methods have to be used to produce them artificially.
Alpha emitters release particles composed of two protons and two neutrons. They are used in cancer therapy because their range in the tissue is very small, but they nonetheless attack and kill cancer cells very effectively thanks to their high energy. Their half-life of seven days in the case of Lutetium-177 and ten in the case of Actinium-225 is ideal for the purpose: it is long enough to enable effective treatment.
New chelator with advantages
So far, there has only been one complexing agent on the market that binds both radionuclides equally well: DOTA. The most frequently used chelator in nuclear medicine is known for its very stable metal complexes. But DOTA has one big disadvantage: only at what are very high temperatures for biochemical conditions, beyond 80 degrees Celsius, is it possible to bond theranostic radionuclides completely. “If you are working with protein derivatives, these temperatures are way too high because even at 40 degrees Celsius denaturation kicks in: they are destroyed. Our new chelator system functions reliably at these lower temperatures,” Kubeil is pleased to report.
Moreover, under these milder conditions, it achieves faster radiolabeling than the known chelators. Another advantage is that the new system efficiently attaches to various bioconjugates. This means an increase in the choice of docking sites on diseased tissue. The newly developed chelator could thus form the basis for new modular and personalized pharmaceutical systems that could be geared towards different fields for imaging and therapy by simply exchanging partial chemical structures.
Publication:
P. Cieslik, M. Kubeil, K. Zarschler, M. Ullrich, F. Brandt, K. Anger, H. Wadepohl, K. Kopka, M. Bachmann, J. Pietzsch, H. Stephan, P. Comba, Toward Personalized Medicine: One Chelator for Imaging and Therapy with Lutetium-177 and Actinium-225, in Journal of the American Chemical Society, 2022 (https://doi.org/10.1021/jacs.2c08438)
Additional information:
Dr. Manja Kubeil
Institute of Radiopharmaceutical Cancer Research at HZDR
Phone : +49 351 260 2442 | Email: m.kubeil@hzdr.de
Media contact:
Simon Schmitt | Head
Department of Communication and Media Relations at HZDR
Phone: +49 351 260 3400 | Mob.: +49 175 874 2865 | Email: s.schmitt@hzdr.de
The Helmholtz-Zentrum Dresden-Rossendorf (HZDR) performs – as an independent German research center – research in the fields of energy, health, and matter. We focus on answering the following questions:
• How can energy and resources be utilized in an efficient, safe, and sustainable way?
• How can malignant tumors be more precisely visualized, characterized, and more effectively treated?
• How do matter and materials behave under the influence of strong fields and in smallest dimensions?
To help answer these research questions, HZDR operates large-scale facilities, which are also used by visiting researchers: the Ion Beam Center, the Dresden High Magnetic Field Laboratory and the ELBE Center for High-Power Radiation Sources.
HZDR is a member of the Helmholtz Association and has six sites (Dresden, Freiberg, Görlitz, Grenoble, Leipzig, Schenefeld near Hamburg) with almost 1,500 members of staff, of whom about 670 are scientists, including 220 Ph.D. candidates.
Contact for scientific information:
Dr. Manja Kubeil
Institut für Radiopharmazeutische Krebsforschung am HZDR
Tel.: +49 351 260 2442 | E-Mail: m.kubeil@hzdr.de
Original publication:
P. Cieslik, M. Kubeil, K. Zarschler, M. Ullrich, F. Brandt, K. Anger, H. Wadepohl, K. Kopka, M. Bachmann, J. Pietzsch, H. Stephan, P. Comba, Toward Personalized Medicine: One Chelator for Imaging and Therapy with Lutetium-177 and Actinium-225, Journal of the American Chemical Society, 2022 (https://doi.org/10.1021/jacs.2c08438)
More information:
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Faster, more energy-efficient way to manufacture an industrially important chemical
Zirconium combined with silicon nitride enhances the conversion of propane — present in natural gas — needed to create in-demand plastic, polypropylene. Polypropylene is a common type of plastic found…

Energy planning in Ghana as a role model for the world
Improving the resilience of energy systems in the Global South. What criteria should we use to better plan for resilient energy systems? How do socio-economic, technical and climate change related…

Artificial blood vessels could improve heart bypass outcomes
Artificial blood vessels could improve heart bypass outcomes. 3D-printed blood vessels, which closely mimic the properties of human veins, could transform the treatment of cardiovascular diseases. Strong, flexible, gel-like tubes…




















