Discovery in Parkinson’s research
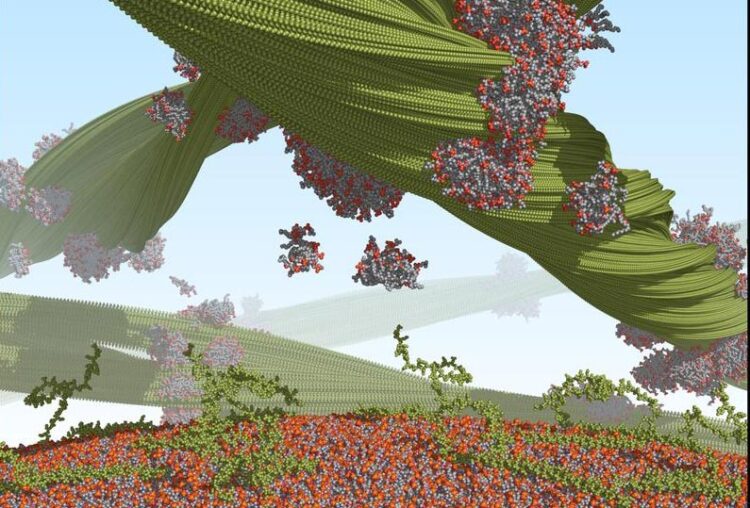
Alpha-synuclein proteins (green) form long helical fibrils (top). During fibril formation, the proteins extract lipids (gray/red) from lipid membranes (bottom) and incorporate these lipids into the fibril structure.
Credit: Benedikt Frieg / Forschungszentrum Jülich
Lipids influence the formation of protein clumps.
After Alzheimer’s, Parkinson’s is the most common neurodegenerative disease in the world. More than six million people worldwide suffer from it. In this disease, alpha-synuclein proteins form thread-like structures called fibrils. When these fibrils clump together, they probably damage nerve cells. A research team has now shown for the first time how lipids bind to the fibril surface and influence the arrangement of the synuclein proteins within the fibrils. As it demonstrated, the drug candidate anle138b binds into a tube-shaped hole inside such a lipidic fibril. The researchers’ findings could open new approaches to diagnosing and treating Parkinson’s disease.
It is a disease with many faces: In the progressive stage of Parkinson’s, limbs begin to tremble, muscles become stiff, movements slow down. In some cases, cognitive disorders or depression occur. There is currently no cure for Parkinson’s, nor for the related diseases Lewy body dementia and multisystem atrophy where alpha-synuclein fibrils also appear.
Conspicuous deposits in the brain
A striking feature of Parkinson’s disease is clumps of alpha-synuclein protein in the brain. Like other proteins, alpha-synuclein consists of long chains of amino acids that must fold correctly in three dimensions to fulfil their functions. In the wrong shape, they can ‘stack up’ into highly ordered fibrils. The fibrils, in turn, can form even larger aggregates. Scientists suspect that the accumulation of misfolded alpha-synuclein protein impairs the function of nerve cells and contributes to their death.
In its correct folding, however, alpha-synuclein is indispensable for nerve cells. It binds to lipid membranes and is involved in transporting vesicles and releasing the messenger substances they carry in nerve cells. “However, lipids also appear to interact with misfolded alpha-synucleins,” reports Gunnar Schröder, group leader at Forschungszentrum Jülich and professor at Heinrich Heine University Düsseldorf. “It has long been suspected that interactions between lipids and misfolded alpha-synuclein proteins could play a role in the development of Parkinson’s disease. But so far, we know very little about this relation,” explains Bert de Groot, research group leader at the Göttingen Max PIanck Institute (MPI) for Multidisciplinary Sciences.
Lipids influence fibril formation
The scientists have now been able to close this knowledge gap. Using cryo-electron microscopy, they succeeded in visualizing for the first time how lipid molecules bind to the fibril surface and connect the units with each other. By using sophisticated computer simulations combined with solid-state nuclear magnetic resonance spectroscopy, the team was also able to show how the lipid and protein elements interact within fibrils.
Surprisingly, several completely novel fibrils formed in the presence of lipids. “Our findings underline that we need to study alpha-synuclein fibrils also in the presence of lipids if we want to understand the molecular basis of alpha-synuclein related pathologies,” Max Planck Director Christian Griesinger comments.
Parkinson’s drug candidate anle138b binds to lipidic fibrils
“The promising drug candidate anle138b also binds to lipidic alpha-synuclein structures. The drug attaches to the tubular cavities within the lipidic fibril,” says Loren Andreas, research group leader at the MPI. “We also find such cavities in other proteins that misfold and are associated with neurodegenerative diseases, for example the tau and the prion protein. The exciting question for us now is whether anle138b attaches there in a similar way and could therefore also provide a diagnostic and therapeutic approach for such diseases.”
Anle138b was developed in 2013 by Griesinger’s team together with Armin Giese and his colleagues from Ludwig Maximilians University in Munich. In tests on mice, the compound successfully delays the progression of Parkinson’s disease. It is currently being further developed by MODAG AG in collaboration with the company Teva. In a clinical phase I study, it proved to be tolerable for humans, which enables further ongoing clinical trials.
Wissenschaftliche Ansprechpartner:
Dr. Loren B. Andreas
Head of the Emmy Noether Research Group Solid-State NMR Spectroscopy
Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany
phone: +49 551 201-2214
e-mail: land@mpinat.mpg.de
Prof. Dr. Gunnar Schröder
Head of the Computational Structural Biology Group
Forschungszentrum Jülich, Jülich, Germany
phone: +49 2461 61-3259
e-mail: gu.schroeder@fz-juelich.de
Originalpublikation:
Frieg, B., Antonschmidt, L., Dienemann, C. et al.: The 3D structure of lipidic fibrils of α-synuclein. Nat Commun, 13, 6810 (2022).
https://doi.org/10.1038/s41467-022-34552-7
Antonschmidt, L., Matthes, D., Dervişoğlu, R. et al. The clinical drug candidate anle138b binds in a cavity of lipidic α-synuclein fibrils. Nat Commun 13, 5385 (2022).
https://doi.org/10.1038/s41467-022-32797-w
Weitere Informationen:
https://www.mpinat.mpg.de/4254758/pr_2233 – Original press release
https://www.mpinat.mpg.de/andreas – Website of Loren Andreas’ Emmy Noether Research Group Solid-State NMR Spectroscopy, Max Planck Institute for Multidisciplinary Sciences, Göttingen
http://www.schroderlab.org/ – Website of Gunnar Schröder’s Computational Structural Biology Group, Forschungszentrum Jülich, Jülich
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A blueprint for mapping melting ice sheets
Researchers in the Stanford Radio Glaciology lab use radio waves to understand rapidly changing ice sheets and their contributions to global sea-level rise. This technique has revealed groundwater beneath Greenland,…

Water hyacinth plant pots – utilization of an invasive species
Together with Fiber Engineering GmbH, the DITF presents a process for the production of biodegradable plant pots. The products are cost effective and competitive. At the same time, the production…

Current research on the new 6G mobile communications standard
Nursing care robots, autonomous driving, digital twins: all of these high-tech applications will play an essential role for the new 6G mobile communications standard. The first commercial 6G networks are…



