Desirable defects
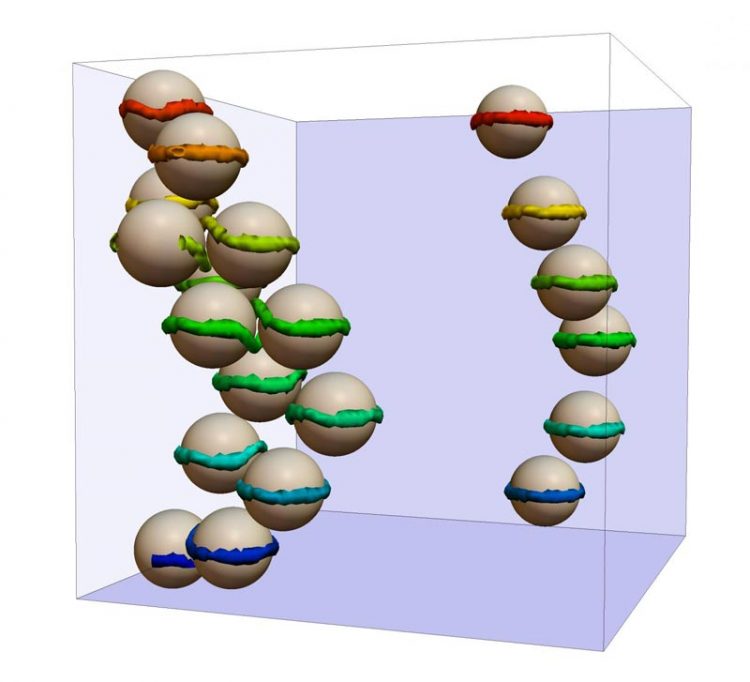
This is a simulation of colloids in liquid crystals. Credit: SISSA
“Generally, flaws are the last thing you'd want in a liquid crystal”, explains Giuseppe D'Adamo, postdoctoral fellow at SISSA. “However, this new method allows us to exploit the defects in the material to our advantage”. D'Adamo is first author of a paper just published in Physical Review Letters.
The study made computer models of colloidal suspensions in liquid crystals subjected to electrical fields modulated over time. Colloids are particles in suspension (i.e., a condition halfway between dispersion and solution) in a liquid.
These composite materials have been receiving plenty of attention for their optical properties for some time now, but the use of electrical fields to modify them at will is an absolute novelty. “Our simulations demonstrate that by switching on or off an electrical field of appropriate intensity we can re-order the colloids by arranging them into columns or planes”, comments Cristian Micheletti of SISSA, co-author of the paper. “This easy-to-control plasticity could make the material suitable for optical-electronic devices such as e-readers, for example”.
Liquid crystals are particular types of liquids. In a normal liquid, molecules have no systematic arrangement and, viewed from any angle, they always appear the same. The molecules forming liquid crystals, by contrast, are arranged in precise patterns often dictated by their shape. To get an idea of what happens in a liquid crystal, imagine a fluid made up of tiny needles which, instead of being arranged chaotically, all point in the same direction. This also means that if we look at the liquid from different viewpoints it will change in appearance, for example it might appear lighter or darker (have you ever seen this happen in LCD monitors, especially the older models?).
“The useful natural tendency of liquid crystal molecules to spontaneously arrange themselves in a certain pattern can be counteracted by introducing colloids in the fluid. In our case, we used microscopic spherical particles, which 'force' the molecules coming into contact with their surface to adapt and rotate in a different direction” explains D'Adamo. “This creates 'defect lines' in the material, i.e., circumscribed variations in the orientation of molecules which result in a local change in the optical properties of the medium”.
More in detail…
These defect lines have an important effect: they enable remote interactions among colloidal particles, by holding them together as if they were thin strings. “Liquid crystal molecules tend to align along the electrical field. By switching the field on and off we create competition between the spontaneous order of the liquid crystal, the order dictated by the surface of the colloidal particles and, finally, the order created by the electrical potential”, says Micheletti. “This competition produces many defect lines that act on the colloids by moving them or clustering them”.
“It's a bit like pulling the invisible strings of a puppet: by carefully modulating the electrical fields we can, in principle, make all the particles move and arrange them as we like, by creating defect lines with the shape we want” continues D'Adamo. “An important detail is that the colloidal configurations are metastable, which means that once the electrical field has been switched off the colloids remain in their last position for a very long time”.
In brief, this implies that the system only requires energy when it changes configuration, a major saving. “In this respect, the method works like the electronic ink used in digital readers, and it would be interesting to explore its applicability in this sense”, concludes Micheletti. The study, carried out with the collaboration of SISSA, the University of Edinburgh and the University of Padova, has been included as an Editors' Suggestion among the Highlights of the journal Physical Review Letters.
###
Useful links:
Original paper: http://arxiv.
IMAGES & VIDEO:
More IMAGES and VIDEO on Flickr: http://goo.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Trotting robots reveal emergence of animal gait transitions
A four-legged robot trained with machine learning by EPFL researchers has learned to avoid falls by spontaneously switching between walking, trotting, and pronking – a milestone for roboticists as well…

Innovation promises to prevent power pole-top fires
Engineers in Australia have found a new way to make power-pole insulators resistant to fire and electrical sparking, promising to prevent dangerous pole-top fires and reduce blackouts. Pole-top fires pose…

Possible alternative to antibiotics produced by bacteria
Antibacterial substance from staphylococci discovered with new mechanism of action against natural competitors. Many bacteria produce substances to gain an advantage over competitors in their highly competitive natural environment. Researchers…