ALS disease mechanism discovered
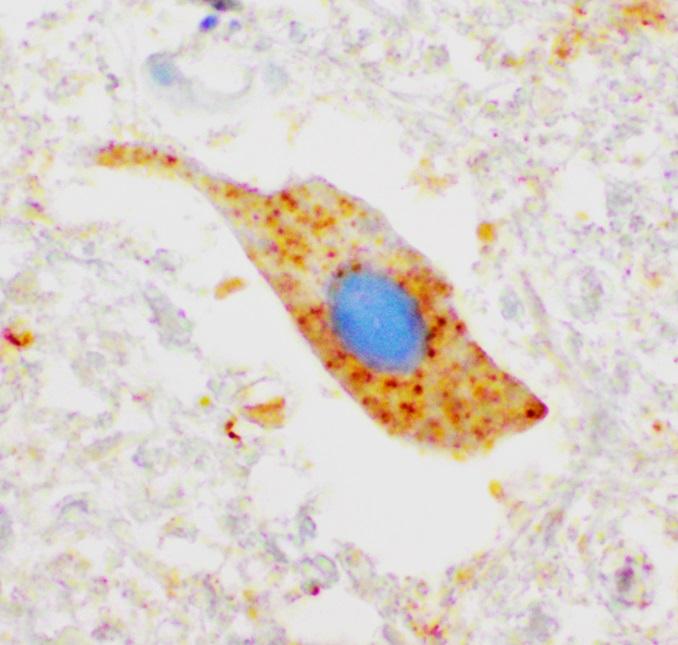
Clumps of SOD1 protein (coloured brown) in the nerve cell that handle the body's motor functions. The image comes from an ALS patient lacking mutation in SOD1. This indicates that the protein is involved in the disease mechanism of ALS even if no mutation occurs in the protein. Photo: Karin Forsberg
“We've been able to identify two different types of protein aggregates with different structures and propagation abilities. One type gave rise to a more aggressive disease progression, which shows that these aggregates are the driving force in the development of ALS,” says Johan Bergh, doctoral student at the Department of Medical Biosciences at Umeå University, Sweden.
Together with the ALS group at Umeå University, Johan Bergh has developed a method of investigating protein aggregates formed in ALS, Amyotrophic lateral sclerosis. With this new method, it has then been possible to identify the particular protein aggregates that are driving in the emergence of ALS.
The protein that has been targeted is superoxid dismutas-1, SOD1. It has long been known that mutations in that protein can cause ALS. The goal of the research team was to investigate the way in which the protein contributes to the disease.
In several diseases afflicting the nervous system, such as in Alzheimer's and Parkinson's Disease, new studies show that some proteins assume an abberant structure. Misfolded proteins aggregate and provoke other proteins of the same kind to assume the same structure. In this way, the disease spreads step by step into the nervous system.
“Using the new method, we have shown and confirmed through animal models that the development of ALS follows the same principle as for other severe nervous disorders. Protein aggregates function as a template that healthy proteins stick to and cause the disease to spread,” says Johan Bergh.
In animal models, agregates of the SOD1 protein from animals, as well as humans, have been shown to induce ALS disease. Amyotrophic lateral sclerosis, ALS, is a fatal neurodegenerative disease which afflicts approximately 250 people annually in Sweden. Although the disease has been known for over 100 years, there is still only one medicine with a disease delaying effect available in Sweden.
“Through our new method, I hope that in the future, drugs will be developed specifically aimed at attacking these protein aggregates. Hopefully, research teams focusing on similar diseases will adopt the method. However, we are in an early phase, and developing drugs is a long-term process,” says Johan Bergh.
Johan Bergh has a degree in biomedicine and is a medical doctor at Umeå University. He is now completing his doctoral studies at the Department of Medical Biosciences, started in 2010.
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















