Zooming-in on protein teamwork
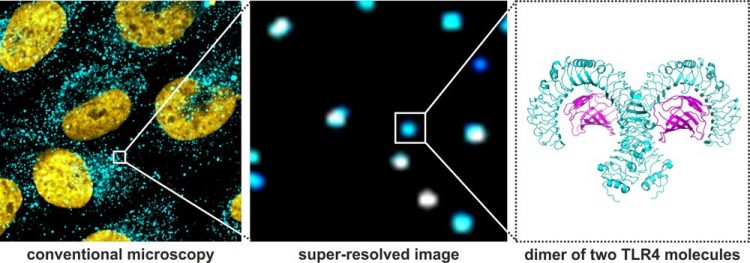
Left: Conventional light microscopy is an useful tool in visualising biological structures and processes. However, its resolution is not sufficient to study events occurring at molecular scale. The image on the left shows the nuclei of brain tumour cells (yellow: nuclei containing DNA) with Toll-like receptors 4 localised at the cell surface (cyan spots). Although many TLR4 can be clearly seen, the spatial resolution does not allow determination of single receptor units. Middle: Super-resolution microscopy greatly improves the spatial resolution and allows detection of single TLR4 clusters (cyan) at the surface of the cells. However, even at this superior resolution, it is not possible to distinguish between monomers and dimers of the receptor. Right: Crystal structure of a TLR4 dimer. The novel analysis method developed by the consortium is able to provide information allowing differentiating between receptor monomers and dimers. Image rights: Widera/Heilemann
The surface of every cell contains receptors that react to external signals similar to a “gate”. In this way, the cells of the innate immune system can differentiate between friend and foe partly through their “toll-like receptors” (TLRs). Two parts of this gate often work together here, as researchers at Goethe University Frankfurt and their British colleagues have now found out with the help of a new super-resolution optical microscopy technique.
When the German Nobel Prize winner Christiane Nüsslein-Volhard discovered receptors in the fruit fly (Drosophila melanogaster) in the 1990s that transduced signals from the cell surface into a cellular response, she was amazed. She nicknamed the receptors “toll” (amazing) and this term has meanwhile become firmly established in scientific literature.
Since then, similar receptors (toll-like receptors) have also been discovered in animals and humans. They recognize bacteria, viruses and fungi and thus ensure that our body reacts to infections in a suitable way. By contrast, de-regulated TLRs can lead to chronic inflammatory conditions and cancer.
Experiments conducted so far indicated that TLRs are activated by a chemical signal that causes two proteins to cluster together as dimers. This process, which is known as “dimerization”, appears to play a pivotal role in a cell’s fate: It can decide whether the cell survives, dies or moves within the body.
Because dimerization takes place on a molecular scale that cannot be captured using conventional microscopy techniques, researchers have to date been dependent on indirect measuring methods. These were, however, prone to error and yielded diverging results. This has now changed thanks to the new super-resolution optical microscopy technique.
In the forthcoming issue of “Science Signaling”, the working groups led by Professor Mike Heilemann of Goethe University Frankfurt and by Dr. Darius Widera and Dr. Graeme Cottrell of the University of Reading in England describe how they have studied the organization of the TLR4 receptor on the cell surface in molecular resolution.
In a first step, they used a super-resolution microscope with a resolution about 100 times better than a standard fluorescence microscope. Since this was still not sufficient to make single receptor molecules in a tiny protein dimer visible, the researchers developed a more sophisticated analysis of the optical signal. In this way they were able to zoom in closer on the super-resolution images and examine under which conditions TLR4 forms a monomer or a dimer. The researchers could also detect which chemical signals from different pathogens modulate the receptors’ patterns.
The researchers hope that their work will lead in future to a better understanding of how TLR dimerization affects the decision between the life or death of a cell. It might also be possible to determine how pharmaceutical ingredients targeted at TLRs influence the behavior of cancer cells.
“It is also conceivable that this approach will help us in future to understand better the fundamental biological processes that regulate the immune system in health and disease. At the same time, this microscopy method is also applicable to other membrane proteins and many similar questions,” explains Professor Mike Heilemann from the Institute of Physical and Theoretical Chemistry at Goethe University Frankfurt.
Publication:
Carmen L. Krüger, Marie-Theres Zeuner, Graeme S. Cottrell, Darius Widera, Mike Heilemann: Quantitative single-molecule imaging of TLR4 reveals ligand-specific receptor dimerization, Science Signaling, doi: 10.1126/scisignal.aan1308
A picture can be downloaded under: http://www.muk.uni-frankfurt.de/68944753
Caption: Left: Conventional light microscopy is an useful tool in visualising biological structures and processes. However, its resolution is not sufficient to study events occurring at molecular scale. The image on the left shows the nuclei of brain tumour cells (yellow: nuclei containing DNA) with Toll-like receptors 4 localised at the cell surface (cyan spots). Although many TLR4 can be clearly seen, the spatial resolution does not allow determination of single receptor units. Middle: Super-resolution microscopy greatly improves the spatial resolution and allows detection of single TLR4 clusters (cyan) at the surface of the cells. However, even at this superior resolution, it is not possible to distinguish between monomers and dimers of the receptor. Right: Crystal structure of a TLR4 dimer. The novel analysis method developed by the consortium is able to provide information allowing differentiating between receptor monomers and dimers.
Image rights: Widera/Heilemann
Further information: Professor Mike Heilemann, Institute of Physical and Theoretical Chemistry, Faculty of Biochemistry, Chemistry and Pharmacy, Riedberg Campus, Tel.: +49(0)69-798- 29736, Heilemann@chemie.uni-frankfurt.de.
Media Contact
More Information:
http://www.uni-frankfurt.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















