New Technology provides a deep view into protein structures
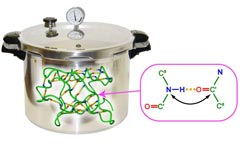
Under pressure: scientists investigate hydrogen bonds under pressures of up to 2500 bar<br>
The specific structure of a protein is stabilized by numerous hydrogen bonds that connect individual amino acids. Using an innovative method, namely Nuclear Magnetic Resonance (NMR) spectroscopy in combination with high pressure, Dr. Nisius and Prof. Grzesiek from the Biozentrum of the University of Basel have provided important new insights into the hydrogen bond network of Ubiquitin and its importance for the stability of this model protein. Their findings have now been published in the renowned scientific journal Nature Chemistry.
Proteins consist of a sequence of amino acids and have important physiological functions, such as catalysis or transport of metabolic products. To perform their physiological role, proteins need to fold their linear amino acid chains into a stable three-dimensional structure. In part, the spatial arrangement is determined by a network of hydrogen bonds. However so far it was unclear to what extent individual hydrogen bonds contribute to the stability of a structure. Using a newly developed high pressure cell and NMR method Dr. Nisius and Prof. Grzesiek could, for the first time, completely characterize the stability of individual hydrogen bonds in the protein Ubiquitin.
Particular stability of key, long-range hydrogen bonds
The stability of a thermodynamic system, such as a protein, can be analyzed by subjecting it to variations in pressure and temperature. Using high resolution NMR methods and a newly developed pressure cell Nisius and Grzesiek have precisely analyzed the contributions of 31 backbone hydrogen bonds to the conformational stability of the model protein Ubiquitin. The pressure cell allows the observation of individual protein hydrogen bonds in the NMR instrument under pressures of up to 2500 bar. The latter is equivalent to the hydrostatic pressure of a water column of 25 km height. Hydrogen bonds spanning small sequence separations between the interacting amino acids were found to be particularly stable, whereas hydrogen bonds that span over larger sequence separations showed generally lower stability. Surprisingly, however, there are exceptions to this rule: hydrogen bonds that connect very important parts of Ubiquitin, can span over large sequence separations and be nevertheless extremely stable. In particular, such unusually stable long-range hydrogen bonds were found in the structural part where Ubiquitin attaches to target proteins. By this covalent attachment, ubiquitin labels misfolded target proteins for degradation and fulfills its function in cellular protein quality control. The specific stabilization of hydrogen bonds at this site is therefore very important to preserve the structural integrity of Ubiquitin during function and to achieve stability for the entire protein.
Future oriented technology: High pressure-NMR
By the high pressure NMR characterization, Nisius and Grzesiek could identify the structural parts of Ubiquitin that are responsible for its unusually high thermodynamic stability. Their study is a further example of the multifaceted and growing range of NMR applications. The technology not only provides information on the three-dimensional structure of biomolecules, but also on their thermodynamic and kinetic characteristics, and thus is a crucial tool to understand biomolecular function at atomic resolution.
Original Article:
Lydia Nisius and Stephan Grzesiek (2012). Key stabilizing elements of protein structure identified through pressure and temperature perturbation of its hydrogen bond network. Nature Chemistry, Published online 8. July, 2012.
Further Information:
Prof. Dr. Stephan Grzesiek, Biozentrum of the University of Basel, Structural Biology & Biophysics, Klingelbergstrasse 50/70, 4056 Basel, Tel: +41 61 267 21 00, E-Mail: stephan.grzesiek@unibas.ch
Media Contact
More Information:
http://www.unibas.chAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















