Scientists pioneer a new way to turn sunlight into fuel
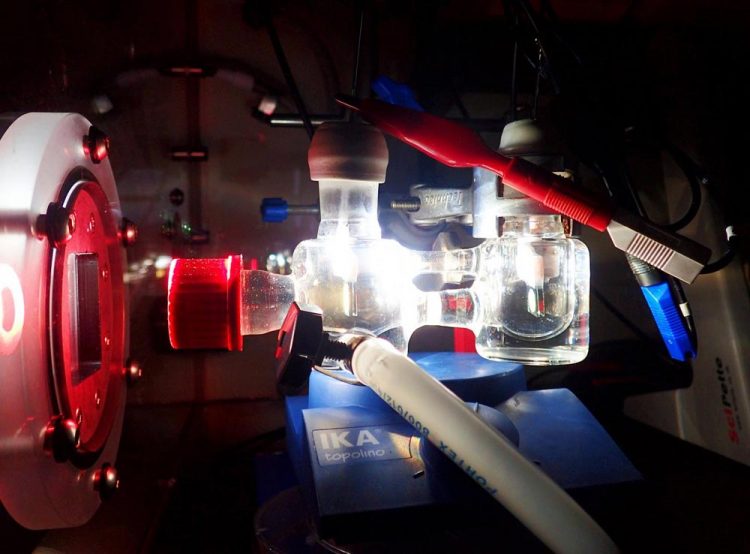
Experimental two-electrode setup showing the photoelectrochemical cell illuminated with simulated solar light. Credit: Katarzyna Sokó?
The quest to find new ways to harness solar power has taken a step forward after researchers successfully split water into hydrogen and oxygen by altering the photosynthetic machinery in plants.
Photosynthesis is the process plants use to convert sunlight into energy. Oxygen is produced as by-product of photosynthesis when the water absorbed by plants is 'split'. It is one of the most important reactions on the planet because it is the source of nearly all of the world's oxygen. Hydrogen which is produced when the water is split could potentially be a green and unlimited source of renewable energy.
A new study, led by academics at St John's College, University of Cambridge, used semi-artificial photosynthesis to explore new ways to produce and store solar energy. They used natural sunlight to convert water into hydrogen and oxygen using a mixture of biological components and manmade technologies.
The research could now be used to revolutionise the systems used for renewable energy production. A new paper, published in Nature Energy, outlines how academics at the Reisner Laboratory in Cambridge developed their platform to achieve unassisted solar-driven water-splitting.
Their method also managed to absorb more solar light than natural photosynthesis.
Katarzyna Sokó?, first author and PhD student at St John's College, said: “Natural photosynthesis is not efficient because it has evolved merely to survive so it makes the bare minimum amount of energy needed – around 1-2 per cent of what it could potentially convert and store.”
Artificial photosynthesis has been around for decades but it has not yet been successfully used to create renewable energy because it relies on the use of catalysts, which are often expensive and toxic. This means it can't yet be used to scale up findings to an industrial level.
The Cambridge research is part of the emerging field of semi-artificial photosynthesis which aims to overcome the limitations of fully artificial photosynthesis by using enzymes to create the desired reaction.
Sokó? and the team of researchers not only improved on the amount of energy produced and stored, they managed to reactivate a process in the algae that has been dormant for millennia.
She explained: “Hydrogenase is an enzyme present in algae that is capable of reducing protons into hydrogen. During evolution this process has been deactivated because it wasn't necessary for survival but we successfully managed to bypass the inactivity to achieve the reaction we wanted – splitting water into hydrogen and oxygen.”
Sokó? hopes the findings will enable new innovative model systems for solar energy conversion to be developed.
She added: “It's exciting that we can selectively choose the processes we want, and achieve the reaction we want which is inaccessible in nature. This could be a great platform for developing solar technologies. The approach could be used to couple other reactions together to see what can be done, learn from these reactions and then build synthetic, more robust pieces of solar energy technology.”
This model is the first to successfully use hydrogenase and photosystem II to create semi-artificial photosynthesis driven purely by solar power.
Dr Erwin Reisner, Head of the Reisner Laboratory, a Fellow of St John's College, University of Cambridge, and one of the paper's authors described the research as a 'milestone'.
He explained: “”This work overcomes many difficult challenges associated with the integration of biological and organic components into inorganic materials for the assembly of semi-artificial devices and opens up a toolbox for developing future systems for solar energy conversion.”
Media Contact
More Information:
http://dx.doi.org/10.1038/s41560-018-0232-yAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















