In the right place at the right time
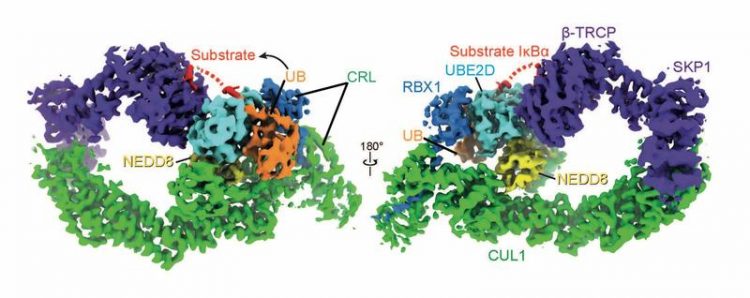
NEDD8 configures the shape of the cullin-RING ligase, UBE2D and ubiquitin so the ubiquitin can be attached to the target substrate. Kheewoong Baek © MPI of Biochemistry
Numerous cellular processes such as immune responses or cell multiplication depend on many different proteins working in sequence. In order for the cell to function correctly, proteins must be degraded after their work is done.
When disease-causing mutations block timely protein degradation, proteins could function at the wrong time, which can lead to diseases, including cancers, heart diseases and developmental disorders.
Controlling protein degradation
Cells “know” to break down proteins by marking unwanted proteins for degradation with another protein called “ubiquitin”. The labelling process, known as ubiquitylation, is carried out by molecular machines, called E3 ligases.
It is important that the E3 ligases themselves are switched on and off in the cells at the right place and at the right time. The “switch on” for about one third of all E3 ligases is a small protein that looks like ubiquitin but is called NEDD8.
NEDD8 at the control of turning off other proteins
Although the individual components of these protein degradation machineries were known, it was unclear how NEDD8 switches on the E3 ligases and enables tagging the target protein with ubiquitin.
“This is especially important because there are drugs in anti-cancer clinical trials that block NEDD8, and some infectious bacteria manipulate NEDD8 to disturb cellular processes”, said Brenda Schulman, head of the “Molecular Machines and Signaling” department at MPIB. Schulman and her team have now deciphered the molecular mechanisms of this ubiquitylation.
“We investigated the mode of action of an E3 turned on by NEDD8. We discovered how NEDD8 induces an E3 molecular machine to bring the ubiquitin tag to its targets. This is a key to switching proteins off at the right time, when no longer needed in a cell,” said Schulman.
Using chemistry and cryo-electron microscopy, the scientists have succeeded in visualizing an important E3 ligase, turned on by NEDD8 and in the process of ubiquitin tagging a target.
“To do this, we took a close look at each step in the tagging process. The natural process occurs within a fraction of a second, after which the molecular tagging machine falls apart. The process of capturing this normally short-lived state was particularly difficult” explains Kheewoong Baek, lead author of the study.
E3 ligase molecular machines control many cellular processes. “The deciphered mechanism not only explains the normal process and what goes wrong in some cancers where mutations prevent the E3 machine from working, but can also serve as a guide for developing therapies to tag unwanted proteins with ubiquitin.
We hope that in the long-term this could help degrade proteins that cause cancer,” summarizes Schulman. [FA]
Prof. Brenda Schulman, PhD
Department Molecular Machines and Signaling
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
E-Mail: schulman@biochem.mpg.de
K. Baek, DT Krist, JR Prabu, S. Hill, M. Klügel, LM Neumaier, S. Gronau, G. Kleiger, BA Schulman: NEDD8 nucleates a multivalent cullin-RING-UBE2D ubiquitin ligation assembly
Nature, February 2020
DOI: https://doi.org/10.1038/s41586-020-2000-y
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















