How proteins regulate the outer envelope of bacterial cells
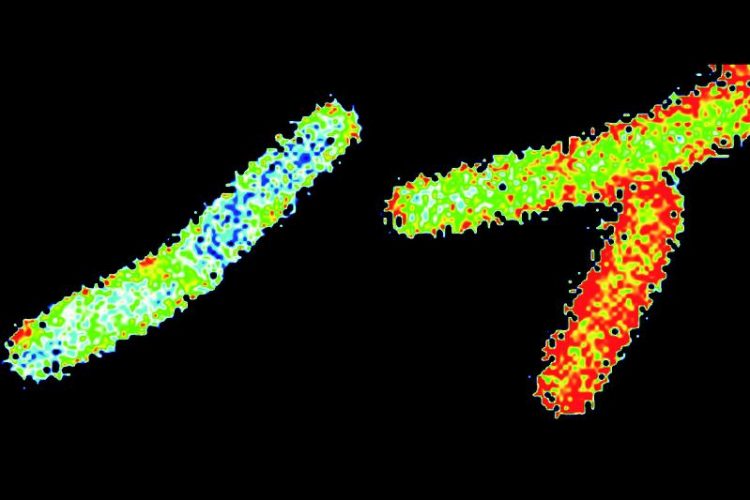
The membrane of Bacillus subtilis cells was marked with the dye Laurdan. The staining indicates the fluidity of the membrane: The membranes of the blue-stained cells are considerably more rigid than the membranes of the reference cells (in red). © Prof. Marc Bramkamp
Like all cells, bacteria have a membrane that shields them from the outside like a skin. This barrier is not static, but has to allow transport of substances in and out and be flexible so that the bacterial cells can grow.
In order to implement these properties, different types of proteins are active in cells, including the so-called flotillins. These proteins are present in cells from bacteria to humans. Until now, scientists assumed that these flotillins mainly help in the formation of other functional protein complexes and confine highly ordered areas of the cell membrane.
A team of international researchers, including researchers from Kiel University, has now found indications of a possible different function of the flotillins: together with colleagues from the Universities of Groningen (the Netherlands) and Bordeaux (France), among others, the Kiel researchers were able to show that flotillin proteins apparently have a direct influence on the structure of the cell membrane and can make it more fluid under certain conditions. The scientists published their findings yesterday in the renowned scientific journal eLife.
Flotillins may work differently than previously assumed
All living cells must be surrounded by a separating barrier that shields them from their environment, but is also permeable for various molecular substances. Various proteins are necessary for the formation of the cell membranes and to equip them with their functions. Until now, researchers assumed that the so-called flotillin proteins serve to contribute to the formation of the necessary functional protein complexes – for example by delimiting certain areas of the membrane.
The work now presented by Professor Marc Bramkamp's Microbial Biochemistry and Cell Biology group at the Institute of General Microbiology at Kiel University contradicts this view:
“Together with a group of international colleagues, we have found evidence that the flotillin proteins have a completely different function. Apparently, they regulate the fluidity of bacterial membranes, making them more fluid to a certain extent and thus, changing their properties,” emphasises Bramkamp.
This assumption might also explain the effect that flotillins have on the synthesis of the cell wall: building blocks for the cell wall are produced inside the cells and must subsequently be “flipped” outwards, which is easier to do in a more fluid membrane. In addition, the protein machinery that synthesises the cell wall moves dynamically through the cell membrane and this movement is significantly reduced in the absence of the flotillins. Consequently, the cells cannot synthesize the cell wall correctly.
Without flotilline no stable form
The Kiel research team developed the new hypothesis in cooperation with international colleagues on the basis of experiments with the rod-shaped bacterium Bacillus subtilis. These experiments showed that fast-growing cells are unable to develop their typical shape in the absence of flotillins.
However, if the researchers added a chemical substance to fluidize the membranes, the bacteria could maintain their shape even without flotillin proteins.
“We therefore assume that they take over a physical role in the bacterial membrane,” emphasises Abigail Savietto, PhD student in Bramkamp's group at Kiel University. “The flotillins seem to have an effect on the physical structure of the membrane, conferring the correct fluidity so other membrane-bound processes can function properly”,” Savietto continues.
In further research, the researchers hope to find out what is the exact molecular mechanism between the flotillin proteins and membrane fluidity. One approach might be the investigation of the phospholipid composition of the membrane. These lipids are involved in the formation of many different biomembranes.
It is possible that the flotillin proteins are able to bind certain phospholipids that reduce fluidity and thus increase the total fluidity of the cell membrane. The new hypothesis of the Kiel research team thus also holds promising perspectives for application: in the future, it might be possible to specifically influence the physical properties of bacterial cell membranes by disrupting the flotillin function.
“My research group has been working on the function of flotillins for many years and we know that cells with altered membrane fluidity are much more sensitive to conventional antibiotics. It might be possible to use this mechanism, for example to specifically alter the membrane of bacterial cells in such a way that they can be killed more easily with antibiotics,” Bramkamp looks ahead.
Photos are available for download:
https://www.uni-kiel.de/de/detailansicht/news/173-zielinska-elife
Caption: Prof. Marc Bramkamp and first author Abigail Savietto investigated how flotillin proteins regulate the fluidity of bacterial membranes.
© Institute of General Microbiology, Kiel University
https://www.uni-kiel.de/de/pressemitteilungen/2020/173-zielinska-elife-authors.j…
Caption: Phase-contrast microscopic image of Bacillus subtilis bacteria in which flotillins were made visible with the aid of the green fluorescent protein GFP.
© Prof. Marc Bramkamp
https://www.uni-kiel.de/de/pressemitteilungen/2020/173-zielinska-elife-comp.jpg
Caption: The membrane of Bacillus subtilis cells was marked with the dye Laurdan and analysed by fluorescence microscopy. The staining indicates the fluidity of the membrane: The membranes of the blue-stained cells are considerably more rigid than the very fluid membranes of the reference cells (in red).
© Prof. Marc Bramkamp
More information:
Institute of General Microbiology, Kiel University:
https://www.mikrobio.uni-kiel.de/de/institut-fuer-allgemeine-mikrobiologie
Prof. Marc Bramkamp
Microbial Biochemistry and Cell Biology
Institute of General Microbiology, Kiel University
Tel.: 0431-880-4341
E-mail: bramkamp@ifam.uni-kiel.de
Aleksandra Zielińska, Abigail Savietto, Anabela de Sousa Borges, Denis Martinez, Melanie Berbon, Joël R. Roelofsen, Alwin M. Hartman, Rinse de Boer, Ida J. van der Klei, Anna K. H. Hirsch, Birgit Habenstein, Marc Bramkamp, Dirk-Jan Scheffers (2020): Flotillin mediated membrane fluidity controls peptidoglycan synthesis and MreB movement. eLife First Published: 14 July 2020
https://doi.org/10.7554/eLife.57179
https://www.mikrobio.uni-kiel.de/de/institut-fuer-allgemeine-mikrobiologie
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















