How cells filter status updates
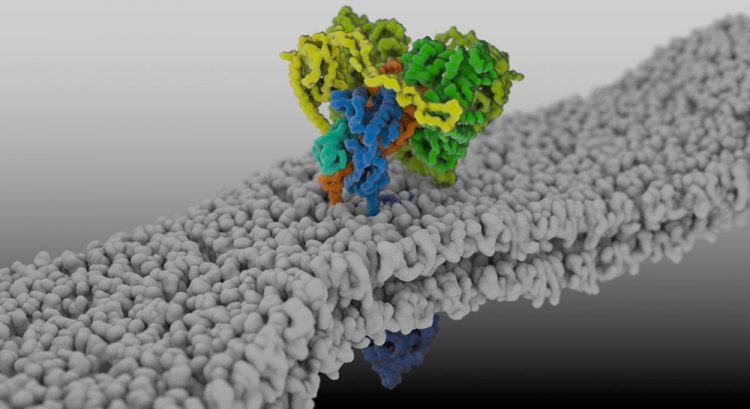
Structure of the MHC-I peptide-loading complex in the membrane of the endoplasmic reticulum. Image rights: Arne Möller (Max Planck Institute of Biophysics), Simon Trowitzsch and Robert Tampé (Goethe University Frankfurt)
Social media have become an indispensable part of our everyday life. We use them constantly to screen the latest news and share pre-selected information. The cells in our body do a similar thing. Information is pre-selected and transmitted to the immune system in order to fight against unwelcome invaders, such as viruses, bacteria, parasites or cancer.
This pre-selection occurs by means of a highly complex molecular machine. Biochemists at Goethe University Frankfurt and the Max Planck Institute of Biophysics, in cooperation with researchers at Martin Luther University Halle-Wittenberg, have now unveiled the inner workings of this complex molecular machine.
Status updates of each cell are transmitted from the cell’s interior to the immune system in the form of small protein fragments. These fragments are presented on the cell surface by specific proteins, known as MHC-I molecules.
Cancerous or infected cells can thus be quickly identified and eliminated. However, viruses and tumours can also trick the immune system and in so doing escape immune surveillance. In addition, ambiguous messages can lead to autoimmune diseases or chronic inflammation.
That is why it is particularly important to understand how this highly complex molecular machine in the cell’s interior selects the relevant protein fragments and coordinates the loading of MHC-I molecules. In the current issue of the renowned scientific journal NATURE, the researchers from Frankfurt and Halle provide first insights into the molecular architecture and inner workings of what is referred to as the MHC-I peptide-loading complex.
“We had to pull out all the stops to prepare this extremely fragile complex for structural analyses,” explains Dr. Simon Trowitzsch of the Institute of Biochemistry at Goethe University Frankfurt. “First of all, we expanded our biochemical toolbox and developed a viral molecular bait that allowed us to isolate the native MHC-I peptide-loading complex from the endoplasmic reticulum.”
“Thanks to groundbreaking advances in cryo-electron microscopy, which were recently awarded the Nobel Prize, we were able to look closely at the MHC-I peptide-loading complex – which is about a hundred thousand times smaller than a pinhead – and to determine its molecular structure,” reports Dr. Arne Möller from the Max Planck Institute of Biophysics.
The scientists can now deduce how the cell manages to generate information important for the immune system. Its structure shows how transport proteins in the membrane, folding enzymes and MHC-I molecules are working together precisely within a highly dynamic complex.
“Our research shows how the MHC-I peptide-loading complex filters out only those fragments of information which are actually needed by the immune system’s effector cells. These findings have solved a decades-old puzzle and allow us now to describe the antigen selection process with greater precision. This knowledge will help to further improve immunotherapies,” concludes Professor Robert Tampé from the Institute of Biochemistry.
Publication: Andreas Blees, Dovilė Janulienė, Tommy Hofmann, Nicole Koller, Carla Schmidt, Simon Trowitzsch, Arne Moeller & Robert Tampé: Structure of the human MHC-I peptide-loading complex, NATURE (Nov 6, 2017, First Release) doi:10.1038/nature24627
A picture can be downloaded from: www.uni-frankfurt.de/69093715
Caption: Structure of the MHC-I peptide-loading complex in the membrane of the endoplasmic reticulum.
Image rights: Arne Möller (Max Planck Institute of Biophysics), Simon Trowitzsch and Robert Tampé (Goethe University Frankfurt)
Further information: Professor Dr. Robert Tampé and Dr. Simon Trowitzsch, Institute of Biochemistry, Faculty of Biochemistry, Chemistry and Pharmacy, Riedberg Campus, Tel.: +49(0)69-798-29475, Tel.: +49(0)69-798-29273, tampe@em.uni-frankfurt.de, Trowitzsch@biochem.uni-frankfurt.de; Dr. Arne Möller, Max Planck Institute of Biophysics, Tel.: +49(0)69-6303-3057, arne.moeller@biophys.mpg.de.
Current news about science, teaching, and society in GOETHE-UNI online (www.aktuelles.uni-frankfurt.de)
Goethe University is a research-oriented university in the European financial centre Frankfurt The university was founded in 1914 through private funding, primarily from Jewish sponsors, and has since produced pioneering achievements in the areas of social sciences, sociology and economics, medicine, quantum physics, brain research, and labour law. It gained a unique level of autonomy on 1 January 2008 by returning to its historic roots as a “foundation university”. Today, it is among the top ten in external funding and among the top three largest universities in Germany, with three clusters of excellence in medicine, life sciences and the humanities. Together with the Technical University of Darmstadt and the University of Mainz, it acts as a partner of the inter-state strategic Rhine-Main University Alliance. Internet: www.uni-frankfurt.de
Publisher: The President of Goethe University Editor: Dr. Anne Hardy, Referee for Science Communication, PR & Communication Department, Theodor-W.-Adorno-Platz 1, 60323 Frankfurt am Main, Tel: (069) 798-13035, Fax: (069) 798-763 12531.
Media Contact
More Information:
http://www.uni-frankfurt.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















