“Hairy” Vehicles in 3D
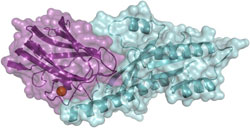
The molecular structure of the two proteins IFT25 and IFT27 forming a macromolecular complex. Picture: Esben Lorentzen / Copyright: MPI of Biochemistry<br>
But the small hair-like structures at the surface of cells can only fulfill these tasks, if their transport system supplies them with all essential building blocks. Scientists at the Max Planck Institute of Biochemistry (MPIB) in Martinsried near Munich, Germany, now managed for the first time to decipher the three-dimensional structure of one part of this complex transport system. That way, they were able to gain important insights into its functional mechanisms. These results can possibly help to prevent pathogenic disruptions. (EMBO Journal, May 19, 2011)
They are situated at the surface of eukaryotic cells and only five to ten micrometers (0.0005 to 0.001 centimeters) long: the cilia. As inconspicuous as these hair-like structures are at first sight, as important are the tasks they fulfill in the body. By distributing specific messenger substances during the development of the embryo, the cilia ensure the correct arrangement of the internal organs. Mistakes in ciliary function can thus result in situs inversus, a condition where the left/right arrangement of the inner organs in the body is reversed. Moreover, motile cilia give the sperm cells mobility and move the egg cells from the ovaries to the uterus along the fallopian tube. Functional disruptions can lead to infertility for men or to a dangerous pregnancy outside the uterus for women. The sensory cilium serves as the antennae of the cell by transmitting signals from the environment and, in doing so, permit different sensory perceptions. These sensory cilia are for example found on photoreceptor cells of the eye and on olfactory neurons. Damage to these types of cilia can thus lead to blindness or the loss of smell.
Although cilia fulfill various tasks, they all have a similar structure: Certain molecules that are essential for the buildup and the preservation of the functioning cilia are transported along a bundle of fibers in the interior of the cilium. Disruption of this transport system, which scientists call intraflagellar transport (IFT), can lead to errors during the assembly of the cilia and thus cause diseases with mental and physical disorders.
Even though the importance of IFT and the cilium to human health has been known for a long time, a structural and mechanistic understanding of IFT has been completely missing. Scientists from the research group of Esben Lorentzen studying “Structural Biology of Cilia” at the MPIB, now succeeded in resolving the structure of a key part of the IFT complex at the molecular level: With the aid of X-ray crystallography, they were able to map this part of the IFT complex in 3D and thus could analyze its structure and functional mechanisms.
“The part of the IFT complex mapped in our study plays an essential role for the regulation of the IFT process. Hence, our findings provide a first step to decipher and understand the structure and the underlying mechanisms of the whole IFT complex”, so says Sagar Bhogaraju, the PhD student at the MPIB who carried out the experiments. In turn, a better understanding of the transport system in the cilium could help to uncover the causes for disruptions and to prevent errors, say the scientists. In this way diseases which occur as a consequence of damaged cilia could potentially be inhibited one day. [UD]
Original Publication:
Bhogaraju, S., Taschner, M., Morawetz, M., Basquin, C. and Lorentzen, E. (2011), Crystal Structure of the Intraflagellar Transport Complex 25/27, EMBO Journal, May 19, 2011.
Contact:
Dr. Esben Lorentzen
Structural Biology of Cilia
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
E-Mail: lorentze@biochem.mpg.de
Anja Konschak
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Tel. +49 89 8578-2824
E-Mail: konschak@biochem.mpg.de
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















