Crouching protein, hidden enzyme
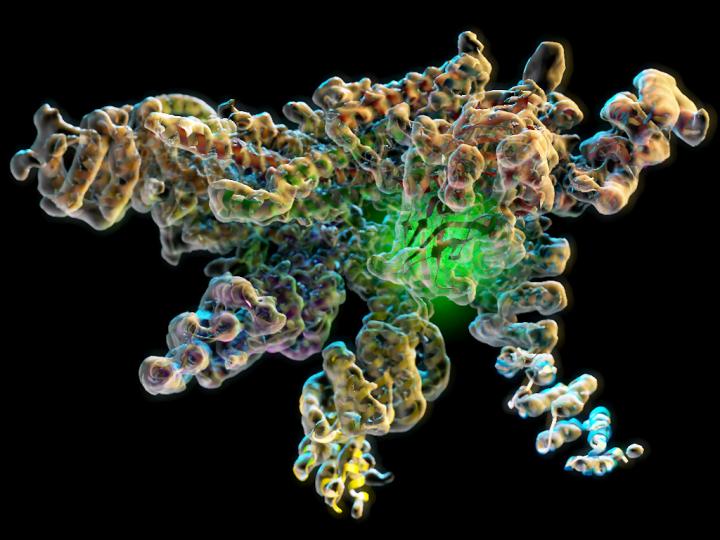
New research from The Scripps Research Institute and UC Berkeley shows the workings of a crucial molecular enzyme. In this image, the green glow in the structure indicates the location of the Rpn11 enzymatic active site in its inhibited conformation at the heart of the isolated lid complex. Credit: Lander lab, The Scripps Research Institute
Meet a microscopic gymnast.
A new study led by scientists at The Scripps Research Institute (TSRI) and the University of California (UC), Berkeley shows how a crucial molecular enzyme starts in a tucked-in somersault position and flips out when it encounters the right target.
The new findings, published recently in the journal eLife, give scientists a clearer picture of the process through which cells eliminate proteins that promote diseases such as cancer and Alzheimer's.
“Having an atomic-resolution structure and a better understanding of this mechanism gives us the ability to someday design therapeutics to combat cancer and neurodegeneration,” said TSRI biologist Gabriel Lander, who was co-senior of author of the study with Andreas Martin of UC Berkeley.
Keeping Cells Healthy
The new study sheds light on the proteasome, a molecular machine that serves as a recycling center in cells. Proteasomes break down spent or damaged proteins and can even eliminate harmful misfolded proteins observed in many diseases.
The new research is the first study in almost 20 years to solve a large component of the proteasome at near-atomic resolution. Lander said the breakthrough was possible with recent advances in cryo-electron microscopy (EM), an imaging technique in which a sample is bombarded with an electron beam, producing hundreds of thousands of protein images that can be consolidated into a high-resolution structure.
Using cryo-EM, scientists investigated part of the proteasome that contains a deubiquitinase enzyme called Rpn11. Rpn11 performs a crucial function called deubiquitination, during which it cleaves molecular tags from proteins scheduled for recycling in the proteasome. This is a key step in proteasomal processing–without Rpn11, the protein tags would clog the proteasome and the cell would die.
From previous studies, scientists knew Rpn11 and its surrounding proteins latch onto the proteasome to form a sort of lid. “The lid complex wraps around the proteasome like a face-hugger in the movie 'Alien,'” said Lander.
The lid complex can also exist separately from the proteasome–which poses a potential problem. If Rpn11 cleaves tags from proteins that haven't gotten to the proteasome yet, those proteins could skip the recycling stage and cause disease. Scientists had wondered how nature had solved this problem.
A Guide for Future Therapies
The study provides an answer, showing the lid complex as it floats freely in cells. In this conformation, Rpn11 is carefully nestled in the crook of surrounding proteins, stabilized and inactive.
“There's a sophisticated network of interactions that pin the Rpn11 deubiquitinase against neighboring subunits to keep it inhibited in the isolated proteasome lid,” explained Corey M. Dambacher, a researcher at TSRI at the time of the study and now a senior scientist at Omniome, Inc., who was first author of the study with TSRI Research Associate Mark Herzik Jr. and Evan J. Worden of UC Berkeley.
“In order for Rpn11 to perform its job, it has to flip out of this inhibited conformation,” said Herzik.
The new study also shows that, to flip out of the conformation at the proteasome, the proteins surrounding deubiquitinase pivot and rotate–binding to the proteasome and releasing the deubiquitinase active site from its nook.
Lander called the system “finely tuned,” but said there may be ways to manipulate it. The study collaborators at UC Berkeley made small mutations to the proteins holding Rpn11 in position, and found that any small change will release the deubiquitinase, even when the lid is floating freely.
Lander said the new understanding of the mechanism that activates Rpn11 could guide future therapies that remove damaged or misfolded proteins.
“Accumulation of these toxic proteins can lead to diseases such as Parkinson's and Alzheimer's, as well as a variety of cancers,” Lander said. “If we can harness the proteasome's ability to remove specific proteins from the cell, this gives us incredible power over cellular function and improves our ability to target certain cells for destruction.”
Going forward, the researchers hope to use the same cryo-EM techniques to investigate other components of the proteasome–and figure out exactly how it recognizes and destroys proteins. “There's still a lot to learn,” said Lander.
###
For more information on the study, “Atomic structure of the 26S proteasome lid reveals the mechanism of deubiquitinase inhibition,” see http://elifesciences.
This research was supported by the Damon Runyon Cancer Research Foundation (grant DFS-#07-13), the Pew Scholars program, the National Institutes of Health (grants DP2 EB020402 and R01-GM094497), the Searle Scholars Program, the National Science Foundation CAREER Program (grant NSF-MCB-1150288), the Howard Hughes Medical Institute and a National Science Foundation Graduate Research Fellowship.
About The Scripps Research Institute
The Scripps Research Institute (TSRI) is one of the world's largest independent, not-for-profit organizations focusing on research in the biomedical sciences. TSRI is internationally recognized for its contributions to science and health, including its role in laying the foundation for new treatments for cancer, rheumatoid arthritis, hemophilia, and other diseases. An institution that evolved from the Scripps Metabolic Clinic founded by philanthropist Ellen Browning Scripps in 1924, the institute now employs about 2,700 people on its campuses in La Jolla, CA, and Jupiter, FL, where its renowned scientists–including two Nobel laureates–work toward their next discoveries. The institute's graduate program, which awards PhD degrees in biology and chemistry, ranks among the top ten of its kind in the nation. For more information, see http://www.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















