Caught in the act
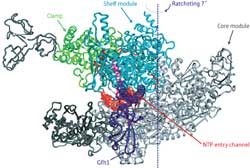
Figure 1: In the RNAP-Gfh1 protein complex the transcription factor (purple) inserts itself in the channel where nucleotides (NTPs) enter (red). This can only happen when the channel has expanded by a ratcheting motion that alters the relative position of the core module (gray) and shelf and clamp modules (light). Copyright : 2011 Shigeyuki Yokoyama<br>
Within the cells, the RNA polymerase (RNAP) protein complex clutches DNA like a crab claw, scanning across gene-coding regions and transcribing these sequences into the messenger RNA molecules that will ultimately provide the blueprint for protein production.
This process can be impaired or assisted through interactions with proteins known as transcription factors, but understanding how these factors influence RNAP function can pose a serious challenge for structural biologists. “It is very difficult to crystallize RNAP, which is an unusually large enzyme,” says Shigeyuki Yokoyama, director of the RIKEN Systems and Structural Biology Center in Yokohama. “In particular, no crystal structures of bacterial RNAP-transcription factor complexes have ever been reported.” Recently, however, Yokoyama and colleagues successfully obtained a crystal structure that captures RNAP in the midst of transcription while bound to Gre factor homologue 1 (Gfh1), a transcription factor from the bacterium Thermus thermophilus[1].
RNAP consists of several discrete modules connected by flexible linker regions, with most of the enzymatic machinery residing in the ‘shelf’ and ‘core’ modules that serve as the main body of the RNAP ‘claw’. In their structure, the researchers uncovered a never-before-seen arrangement of the RNAP modules, where some sort of ‘ratcheting’ action has created notable displacement between the shelf and core relative to its normal structure.
In fact, the binding of Gfh1 appears to lock RNAP into this configuration. This transcription factor—a known inhibitor—inserts itself into a channel on the complex that normally accepts nucleotides for addition onto newly synthesized RNA molecules (Fig. 1). However, such insertion would be impossible with the normal RNAP complex, where the channel is too narrow. This suggests that RNAP executes this unexpected ratcheting motion as part of its normal behavior, which in turn leaves it vulnerable to Gfh1 inhibition. “This conformational change was most surprising,” says Yokoyama. “It was simply impossible to predict this before the structure of RNAP-Gfh1 was solved.”
In subsequent biochemical experiments, he and his colleagues managed to essentially catch RNAP in the act of ratcheting, providing further evidence that this behavior occurs spontaneously in nature and is likely to contribute directly to the enzyme’s transcriptional activity. “We hypothesize that RNAP uses this ratcheted state to slide along DNA chains as an intermediate step in the course of normal transcription,” says Yokoyama. “This state may also be used an intermediate for transcriptional termination, in which the [RNA] dissociates from the RNAP.” He adds that validating these and other hypotheses will be top priorities for future experimental efforts.
The corresponding author for this highlight is based at the RIKEN Systems and Structural Biology Center
Journal information
[1] Tagami, S., Sekine, S., Kumarevel, T., Hino, N., Murayama, Y., Kamegamori, S., Yamamoto, M., Sakamoto, K. & Yokoyama, S. Crystal structure of bacterial RNA polymerase bound with a transcription inhibitor protein. Nature 468, 978–982 (2010).
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Making diamonds at ambient pressure
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions. Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods?[2]…

Eruption of mega-magnetic star lights up nearby galaxy
Thanks to ESA satellites, an international team including UNIGE researchers has detected a giant eruption coming from a magnetar, an extremely magnetic neutron star. While ESA’s satellite INTEGRAL was observing…

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…





















