Weizmann institute scientists solve the 3-D structure of the enzyme involved in Gaucher disease
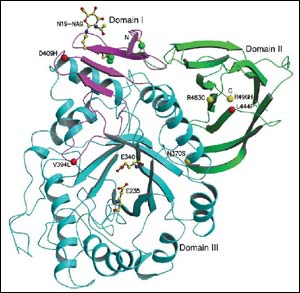
Glucocerebrosidase
Discovery may help design effective therapies for the genetic disease that mainly affects Ashkenazi Jews
An interdisciplinary team of Weizmann Institute scientists has solved the three-dimensional structure of an enzyme called glucocerebrosidase. Mutations occurring in this enzyme cause Gaucher disease, a genetic illness that mainly affects Ashkenazi Jews. The Institute study, published recently in EMBO Reports, may lead to the design of effective new therapies for treating the disease.
Gaucher disease was first described in 1882 by the French physician Philippe Gaucher. It is characterized by swelling and enlargement of the spleen and liver and disruption in the function of these organs, and in rare cases it also affects the brain. In the 1920s, the disease was found to be caused by the excessive accumulation of a fatty substance, or lipid, called glucosylceramide. In the 1960s, researchers discovered that the accumulation occurs due to a defect in the glucocerebrosidase enzyme, whose function is to break down this lipid and regulate its amount. In the 1980s, the gene responsible for manufacturing the enzyme was isolated; scientists found that mutations in this gene disrupt the function of the enzyme, leading to the development of Gaucher disease.
By the early 1990s, the U.S. company Genzyme started producing the enzyme – first from placenta, then by genetic engineering. Today thousands of Gaucher patients are treated by injections of the enzyme, an approach called enzyme replacement therapy, or ERT. The annual cost of the therapy per patient is approximately $100,000 to $300,000. Obviously, more affordable alternatives, such as the ones that may emerge from the Weizmann Institute study, are urgently needed.
The Institute team included Prof. Tony Futerman of the Biological Chemistry Department, Prof. Joel Sussman of the Structural Biology Department and Prof. Israel Silman of the Neurobiology Department, as well as Dr. Michal Harel, Lilly Toker and graduate student Hay Dvir.
The first step in solving the three-dimensional structure of an enzyme is to grow its crystals. In the case of the glucocerebrosidase enzyme involved in Gaucher disease, the crystallization was a formidable challenge. The Weizmann Institute scientists succeeded in this task by cutting parts of certain sugar molecules on the surface of the enzyme.
The scientists then resorted to X-ray crystallography, a method in which the crystal is exposed to X-rays and the structure of its molecules is determined by the diffraction pattern of the X-rays. The X-ray data were collected at the European Synchrotron Radiation Facility in Grenoble, France.
The solution of the enzyme structure may lead to the development of new therapies for Gaucher disease. First, the structural information may help design a more effective enzyme that will improve today’s ERT therapy. This approach is most likely to provide effective additional treatments for Gaucher disease, until the development of gene therapy for this disorder is developed. Another type of therapy likely to emerge from the Weizmann findings is the design of small molecules that will supplement the damaged enzyme in the patient’s body, thereby restoring its normal functioning.
Yeda Research and Development Co., which is responsible for the commercialization of Weizmann Institute research, has filed a patent application for the medical applications of these findings.
Prof. Futerman’s research is supported by The Estate of Ernst and Anni Deutsch-Promotor Stiftung, Switzerland, Paul Godfrey Foundation, and Buddy Taub Foundation. Prof. Futerman is the incumbent of the Joseph Meyerhoff Professorial Chair of Biochemistry.
Prof. Sussman’s research is supported by the Charles A. Dana Foundation, Jean and Jula Goldwurm Memorial Foundation, Helen & Milton A. Kimmelman Center for Biomolecular Structure & Assembly, Joseph and Ceil Mazer Center for Structural Biology, and The late Sally Schnitzer. Prof. Joel Sussman is the incumbent of the Morton and Gladys Pickman Chair in Structural Biology.
Prof. Israel Silman’s research is supported by Nella and Leon Benoziyo Center for Neurosciences, Charles A. Dana Foundation, Carl and Micaela Einhorn-Dominic Brain Research Institute, Helen & Milton A. Kimmelman Center for Biomolecular Structure & Assembly. Prof. Silman is the incumbent of the Bernstein-Mason Professorial Chair of Neurochemistry.
The Weizmann Institute of Science in Rehovot, Israel, is one of the world’s top-ranking multidisciplinary research institutions. Noted for its wide-ranging exploration of the natural and exact sciences, the Institute is home to 2,500 scientists, students, technicians and supporting staff. Institute research efforts include the search for new ways of fighting disease and hunger, examining leading questions in mathematics and computer science, probing the physics of matter and the universe, creating novel materials and developing new strategies for protecting the environment.
Media Contact
More Information:
http://www.weizmann.ac.il/All latest news from the category: Interdisciplinary Research
News and developments from the field of interdisciplinary research.
Among other topics, you can find stimulating reports and articles related to microsystems, emotions research, futures research and stratospheric research.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…