Collagen: powerful workout with water
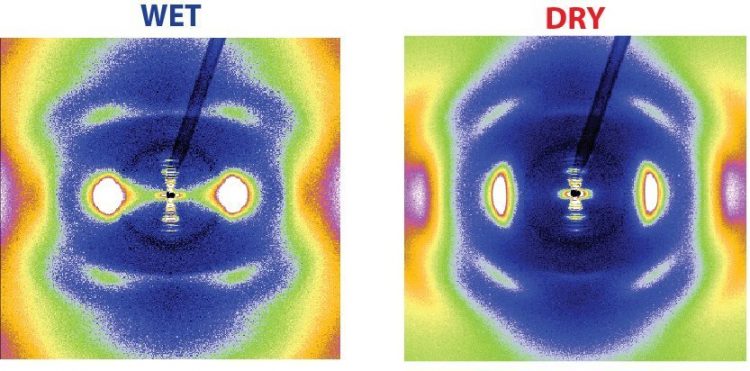
X-ray view of collagen: from the patterns of the two-dimensional X-ray diffraction, information about changes in the molecular and nanoscopic collagen structure can be gained when the protein dries. The structure of collagen is crucial for power generation. © Nature Communications 2015 / MPI of Colloids and Interfaces
The bodies of humans and animals owe their strength especially to a fibrous structural protein called collagen. Collagen is abundant in bones, tendons, ligaments and skin. Water, a substance that is not often associated with strength, was found out to be an intrinsic component of collagen, as researchers at the Max Planck Institute of Colloids and Interfaces in Potsdam-Golm, together with the scientists from the Massachusetts Institute of Technology in Cambridge (USA), have shown.
The team, led by Admir Masic and Luca Bertinetti, unveiled that removing water from collagen fibres has dramatic effects on molecular and nanoscopic features. The fibres contract and generate tensile forces that are 300-times higher than those exerted by human muscles. These findings could help researchers develop novel materials and also suggest that collagen may have more active role in living organisms than previously thought. In fact, it does not act merely as a stabilising framework for the body, but can also generate tensions, for example during the synthesis of bones.
Like a building, collagen has a hierarchical structure consisting of a complex arrangement of individual molecular components. The basic building block is the collagen molecule itself. Its shape reminds of a rope, with three chain-like proteins twisted around each other to form a triple helical motif. Many of these “ropes” in turn combine to form thicker “coils”, known as collagen fibrils.
However, being just 100 to 500 nanometres thick, the fibrils are 100,000 thinner than actual ropes. Within the fibrils, adjacent collagen molecules are not simply stacked one adjacent to each other but they are laid to form a staggered arrangement. This results in alternating denser and thinner zones along the length of the fibrils. Many fibrils in turn combine to form collagen fibres.
Scientists at the Max Planck Institute of Colloids and Interfaces in Potsdam-Golm have now investigated the characteristics of collagen and specifically how its function is influenced by the water content. What makes the study of the Golm-based team so unique is that, for the first time, it combined multiple methods to investigate collagen at the various levels of its hierarchical structure while controlling at the same time the water content of the natural material in a humidity chamber. A special device in the chamber measured the tensile forces acting on collagen obtained from rat tails.
Water is an intrinsic component of collagen
The results obtained in Golm were compared with computer models of collagen fibrils developed by scientists at the Massachusetts Institute of Technology in Cambridge, USA. Using this approach, the researchers were able to investigate in detail the effects of water on the collagen structure.
“Water is an integral component of collagen,” says Admir Masic. In the protein’s natural state, water accounts for about 60 percent of the substance by weight. Water molecules bind tightly to collagen that they follow its helix shape, says Masic. The team discovered this using X-ray diffraction, which revealed details of the helical structure, as for example the angle of the turns and the diameter of the helix.
Given the high content of water in collagen, it is not surprising that its removal has dramatic effects. If the relative humidity is reduced from 95 to 5 percent, the collagen practically dries out, the collagen molecules shorten by 1.3 percent and the corresponding fibrils by 2.5 percent. Despite this relatively small change in length, a tensile force of around 100 megapascals is generated – some 300 times more than that produced by contractile muscle.
Dense regions of the fibrils elongate, whereas thin regions contract
Masic’s and Bertinetti’s team also identified the mechanism underlying this contraction. They used Raman spectroscopy to investigate the conformation of molecular chains of collagen. Conformation describes the relative positions of atoms with respect to each other in a molecule. The researchers found that contraction is caused by these conformational changes. This can be visualised by imagining a rope that is initially straight and shortens by forming wave-like patterns so that its ends are closer together. An interesting detail of the mechanism is that the denser regions of the fibrils elongate, while the thinner regions shorten. The net effect is contraction.
“With this knowledge, researchers could develop materials that behave in a opposite ways when water is removed from them,” says Luca Bertinetti. He describes, for example, two sheet-like materials that are bonded together, one of which expands while the other shrinks upon removal of water. The double layer would then bend. The team’s results show that such materials would be able to exert large forces. The new findings could also be useful for the fabrication of leather and parchments and their preservation.
Potential and still unexplored active function of collagen fibrils
However, the results from Golm are not just interesting from an engineering point of view. Although such massive dehydration as carried out in the researchers’ humidity chamber does not occur in the body of a living organism under physiological conditions, Masic’s and Bertinetti’s team found that the removal of water can be large enough, even under biological conditions, for collagen to generate as much tensile force as human muscles.
The biomolecule could therefore also play an active role rather than a purely passive elastic one – namely in mechanically stabilising the body. “During bone synthesis, water may be removed from collagenous matrix so that the tissue contracts” says Peter Fratzl, the Director of the Institute and the coordinator of the project. Consequently, the bone would be compressed, thus preventing the mineral part, which is actually quite brittle, from being raptured by tensile stresses. The steel in reinforced concrete plays a similar role, says Fratzl.
This assumption is supported by the fact that the spacing between the dense zones of collagen fibrils in bone tissue is the same as that in dry collagen and that the tensile strength of bone corresponds approximately to the tensile strength of dried collagen.
In the near future, the Golm-based researchers plan to investigate the possible physiological role of collagen contraction in various tissues.
Contact
Dr. Admir Mašić
Max Planck Institute of Colloids and Interfaces, Potsdam-Golm
Phone: +49 331 567-9419
Email: admir.masic@mpikg.mpg.de
Prof. Dr. Peter Fratzl
Max Planck Institute of Colloids and Interfaces, Potsdam-Golm
Phone: +49 331 567-9401
Fax: +49 331 567-9402
Email: gabbe@mpikg.mpg.de
Original publication
Admir Masic, Luca Bertinetti, Roman Schuetz, Shu-Wei Chang, Hartmut Metzger, Markus J. Buehler & Peter Fratzl
Osmotic pressure induced tensile forces in tendon collagen
Nature Communications; 22 January 2015; DOI: 10.1038/ncomms6942
Media Contact
More Information:
http://www.mpg.de/8887201/collagen-tendons-bonesAll latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















