Shedding light on the reaction mechanism of PUVA light therapy for skin diseases
Reaction stages when a psoralen molecule binds to DNA. Result: the psoralen is permanently bound to the DNA via a cyclobutane ring. The cell is thus damaged, and triggers the programmed cell death. ACS / Janina Diekmann
Together with their Munich-based colleagues, a team of physical chemists from Heinrich Heine University Düsseldorf (HHU) has clarified which chemical reactions take place during PUVA therapy. The therapy involves light-induced damage to the DNA of diseased cells.
The team working under Prof. Dr. Peter Gilch has now published its findings in the Journal of the American Chemical Society.
The term ‘PUVA’ stands for ‘psoralen’ and ‘UV-A radiation’. Psoralens are natural plant-based compounds that can be extracted from umbelliferous plants such as giant hogweeds. Plant extracts containing psoralens were already used in Ancient Egypt for the treatment of skin diseases.
Modern medical use began in the 1950s. From then on, they were applied for light-dependent treatment of skin diseases such as psoriasis and vitiligo. From the 1970s onwards, PUVA therapy was used to treat a type of skin cancer known as cutaneous T-cell lymphoma.
Psoralens insert between the crucial building blocks (bases) of DNA, the hereditary molecule. When subjected to UV radiation, they bind to thymine – a specific DNA base – and thus cause irreversible damage to the hereditary molecule. This in turn triggers programmed cell death, ultimately destroying the diseased cell.
Researchers working with Prof. Dr. Peter Gilch from HHU’s Institute of Physical Chemistry have now collaborated with Prof. Dr. Wolfgang Zinth’s work group from LMU Munich to analyse the precise mechanism of this binding reaction. They used time-resolved laser spectroscopy for this purpose.
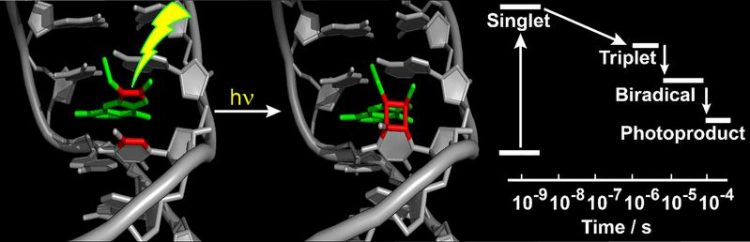
They found that – after the psoralen molecule has absorbed UV light – the reaction takes place in two stages. First, a single bond between the psoralen molecule and thymine forms. A second bond formation then yields a four-membered ring (cyclobutane) permanently connecting the two moieties (see figure).
The researchers in Düsseldorf and Munich were also able to demonstrate that the first stage takes place within a microsecond, while the second needs around 50 microseconds. They compared this process with the damaging of the ‘naked’ DNA by UV light. That process also frequently results in cyclobutane rings, but the process takes place considerably faster than when psoralens are present.
Prof. Gilch explains the background to the research: “If we can understand how the reactions take place in detail, we can change the psoralens chemically in a targeted way to make PUVA therapy even more effective.” Together with his colleague in organic chemistry, Prof. Dr. Thomas Müller, he wants to develop these high-performance psoralen molecules at HHU within the scope of a DFG project.
Janina Diekmann, Julia Gontcharov, Sascha Fröbel, Christian Torres Ziegenbein, Wolfgang Zinth, Peter Gilch, The Photoaddition of a Psoralen to DNA proceeds via the Triplet State, Journal of the American Chemical Society, 2019
DOI: 10.1021/jacs.9b06521
Media Contact
More Information:
http://www.hhu.de/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Sea slugs inspire highly stretchable biomedical sensor
USC Viterbi School of Engineering researcher Hangbo Zhao presents findings on highly stretchable and customizable microneedles for application in fields including neuroscience, tissue engineering, and wearable bioelectronics. The revolution in…

Twisting and binding matter waves with photons in a cavity
Precisely measuring the energy states of individual atoms has been a historical challenge for physicists due to atomic recoil. When an atom interacts with a photon, the atom “recoils” in…

Nanotubes, nanoparticles, and antibodies detect tiny amounts of fentanyl
New sensor is six orders of magnitude more sensitive than the next best thing. A research team at Pitt led by Alexander Star, a chemistry professor in the Kenneth P. Dietrich…





















