Atomic anchors to quicken computer boot-up
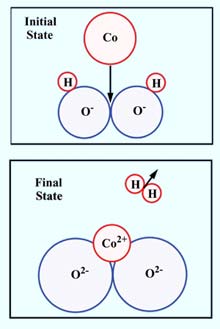
THE ESSENTIAL REACTION. The transition occurs when the impacting cobalt atom is close to where two oxygen atoms, part of two OH-groups, touch. <br>
Simple method may improve catalysts, nanodevices
A way to help next-generation computers boot up instantly, making entire memories immediately available for use, has been developed by researchers at Sandia National Laboratories and Pacific Northwest National Laboratory.
The patented technique is able to deposit flat, ultrathin metallic layers on very thin oxide layers. The thinness of the deposition reduces material cost and requires less electricity to produce more rapid magnetic effects in the service of computer memory.
The inexpensive innovation also may produce better, less expensive catalysts for chemical reactions, better ceramic/metal seals, and lead to improved nanodevices.
The method, reported in the Aug. 2 Science, was discovered at the Pacific Northwest National Laboratory and understood and generalized by theoretical scientists in Sandia’s Surface and Interface Sciences Department.
The technique eliminates the present-day hurdle of metal atoms clustering together into three-dimensional islands when deposited on oxide surfaces. These thick bumps of metal – similar to water beads on a waxed car – are a problem because they produce poorly crystallized metal films. These are relatively weak, require inefficiently large amounts of material, and produce more heat because more electricity is needed to produce variations in magnetic signals.
The new method achieves crystallinity with only a few atomic layers. Its inherent structural strength should also produce greater durability in electronic devices.
The discovery also should enable the production of catalysts where the reactive metal on an oxide support is only one atomic layer thick, thereby saving considerable cost in materials. Catalysts are involved in approximately two-thirds of the gross domestic product of the United States, particularly with regards to oil.
How does the process work?
The findings may have the most immediate bearing on magnetic tunnel junctions, slated for use in magneto-resistive random access memory, or MRAM. MRAM will allow computers to store information in a nonvolatile fashion, meaning that the information is not lost when the computer is turned off. As a result, MRAM promises a day when computers would boot up instantly once turned on, rather than comparatively slowly while retrieving information from the hard drive. Major corporations have begun developing MRAM modules in hopes of generating robust nonvolatile memory in the next few years.
But growing an atomically flat, ultrathin film of metal on top of any insulator material is a difficult feat. In a magnetic tunnel junction, an ultrathin layer of insulator, typically aluminum oxide with a thickness of less than or about 1 nanometer, is sandwiched between layers of magnetic metal, such as cobalt or nickel-iron. When current flows through the device, the magnetic orientation of the two metal layers can be switched, resulting in different values of the tunneling current. This creates an environment in which “bits” of computer memory can be stored.
To achieve ferromagnetism, it was thought that thick layers of the top metal must be made. Obviously, thinner layers that require lower currents to switch the direction of magnetic fields would be more desirable.
In 2000, Sandia solid-state theorist Dwight Jennison approached Scott Chambers, a chief scientist at Pacific Northwest National Laboratory, with a theory that the presence of hydroxyls – in effect, water fragments – would enhance the binding of metals to oxide surfaces. Jennison calculated that then certain metals would form flat films on sapphire (a phase of aluminum oxide).
Using a special synthesis technique he created, Chambers and postdoctoral fellow Tim Droubay produced an atomically flat film of cobalt on hydroxylated sapphire. They found, as Jennison had suspected, that the cobalt accumulated in layer-by-layer fashion, rather than clustering to form islands.
“Cobalt’s interaction with oxide is so weak that it would normally ball up when deposited,” says Jennison. “However, by changing the surface of the oxide, Scott discovered that cobalt atoms can cause the release of a hydrogen gas molecule and the cobalt atoms then become oxidized themselves – that is, they link up with the newly available oxygens and end up strongly bound within the top layer of the oxide. These are the anchors.”
These metal atoms, embedded at scattered points within the top layer of the oxide, amount to about one anchor for every ten oxygen atoms in the top layer. These anchoring atoms bind other metallic atoms to themselves and to each other just above the oxide surface, forming a crystalline metallic layer.
“Many advanced technologies rely on strong interfaces between metals and oxides,” said Chambers, lead author of the Science paper. “These findings may provide the molecular insight industry needs to create better materials for microelectronics and sensors.”
The new technique uses equipment already in place in chip manufacturing plants.
“For industry, a solution may be as simple as exposing the thin aluminum oxide films to a low pressure of water vapor before adding a final cobalt layer,” said Chambers. The entire process may be done at room temperature, which is beneficial since it is often important to avoid high temperatures in manufacturing.
PNNL postdoc Tim Droubay helped Chambers with the experiments. Jennison, who first found which chemical reactions would be energetically favorable, collaborated at Sandia with Thomas Mattsson, who has long experience in first-principle-based diffusion and reaction studies, and in computing critical reaction barriers. Their theoretical first principles calculations predicted some and validated other experimental results. Some of these calculations required work on Sandia’s most powerful computers.
The calculations provided insight into what reaction is taking place, where it occurs, the energy barrier for it to happen, and the time needed for completion vs. the time for arriving cobalt atoms to lose energy while in contact with the surface. If the reaction occurred slowly, the rapidly diffusing cobalt atoms would first find a growing island. However, because hydrogen molecules – the lightest of all molecules – are being made, the reaction can be on the order of tenths of a picosecond. This is well before the arriving cobalt atoms can assume the temperature of the substrate.
Says Jennison, “Otherwise the experimental result would be impossible to explain. However, here we have a wonderful joining of theory and experiment.”
While the experiment was conducted using cobalt, Jennison’s calculations predict the method also would be effective for iron and nickel, two other metals under consideration for MRAM, as well as metals such as copper, ruthenium, and rhodium. The latter two have applications in catalysis.
Pacific Northwest National Laboratory is a DOE research facility and delivers breakthrough science and technology in the areas of environment, energy, health, fundamental sciences and national security. Battelle, based in Columbus, Ohio, has operated the laboratory for DOE since 1965. The Division of Material Science within the DOE’s Office of Basic Energy Sciences supported the research at PNNL.
Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin company, for the U.S. Department of Energy’s National Nuclear Security Administration. With main facilities in Albuquerque, N.M., and Livermore, Calif., Sandia has major R&D responsibilities in national security, energy and environmental technologies, and economic competitiveness.
Sandia Media Contact: Neal Singer, (505) 845-7078, nsinger@sandia.gov
PNNL Media Contact: Staci Maloof, (509) 372-6313, staci.maloof@pnl.gov
Story and images available at www.sandia.gov/news-center/news-releases/2002/gen-science/atomicanchor.html
Sandia National Laboratories
A Department of Energy National Laboratory
Managed and Operated by Sandia Corporation
ALBUQUERQUE, NM LIVERMORE, CA
MEDIA RELATIONS DEPARTMENT MS 0165
ALBUQUERQUE, NM 87185-0165
PHONE: (505) 844-8066 FAX: (505) 844-0645
Media Contact
All latest news from the category: Process Engineering
This special field revolves around processes for modifying material properties (milling, cooling), composition (filtration, distillation) and type (oxidation, hydration).
Valuable information is available on a broad range of technologies including material separation, laser processes, measuring techniques and robot engineering in addition to testing methods and coating and materials analysis processes.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















