A Theoretical Breakthrough Inspired by Experiment
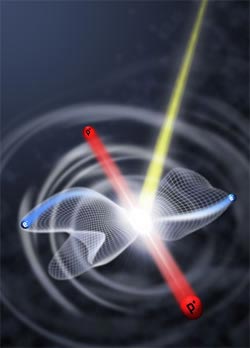
A hydrogen molecule hit by an energetic photon breaks apart. The ejected electrons, blue, take paths whose possible trajectories (here represented by net-like lobes) depend on how far apart the hydrogen nuclei, red, are at the moment the photon strikes. The bond length at that instant reflects how the molecule’s electrons are correlated. (Courtesy Wim Vanroose)
Calculating Electron Correlations in the Hydrogen Molecule
Need to understand the details of how a molecule is put together? Want to see the effects of the intricate dance that its electrons do to make a chemical bond? Try blowing a molecule to bits and calculating what happens to all the pieces. That’s the approach taken by an international group of collaborators from the University of California at Davis, universities in Spain and Belgium, and the Chemical Sciences Division of the Department of Energy’s Lawrence Berkeley National Laboratory.
A hydrogen molecule hit by an energetic photon breaks apart. The ejected electrons, blue, take paths whose possible trajectories (here represented by net-like lobes) depend on how far apart the hydrogen nuclei, red, are at the moment the photon strikes. The bond length at that instant reflects how the molecule’s electrons are correlated. (Courtesy Wim Vanroose)
When a hydrogen molecule, H2, is hit by a photon with enough energy to send both its electrons flying, the two protons left behind — the hydrogen nuclei — repel each other in a so-called Coulomb explosion. In this event, called the double photoionization of H2, the paths taken by the fleeing electrons have much to say about how close together the two nuclei were at the moment the photon struck, and just how the electrons were correlated in the molecule.
Correlation means that properties of the particles like position and momentum cannot be calculated independently. When three or more particles are involved, calculations are notoriously intractable, both in classical physics and quantum mechanics. In the 16 December, 2005 issue of Science the researchers report on the first-ever complete quantum mechanical solution of a system with four charged particles.
The groundbreaking calculations were inspired by earlier experiments on the photofragmentation of deuterium (heavy hydrogen) molecules, performed at beamline 9.3.2 of Berkeley Lab’s Advanced Light Source (ALS) in 2003 by a group of scientists from Germany, Spain, and several institutions in the United States. The experimenters were led by Thorsten Weber, then with the ALS and now at the University of Frankfurt.
“If you were trying to do this experiment and you didn’t have access to the Advanced Light Source and a COLTRIMS experimental device” — a sophisticated, position-sensitive detector for collecting electrons and ions — “you’d just fire photons at a random sample of hydrogen molecules and measure the electrons that came out,” says Thomas Rescigno of Berkeley Lab’s Chemical Sciences Division, one of the authors of the Science paper. “What made this experiment special was that they could measure what happened to all four particles. From their precise positions and energy they could reconstruct the state of the molecule when it was hit.”
Weber presented early experimental data at a seminar attended by Rescigno, William McCurdy of the Lab’s Chemical Sciences Division, who is also a professor of chemistry at the University of California at Davis, and Wim Vanroose, a postdoctoral fellow at Berkeley Lab who is now at the Department of Computer Science at the Katholieke Universiteit Leuven in Belgium.
Says Rescigno, “Thorsten teased us with his results, some of which were extremely nonintuitive. What was remarkable was that very small differences in the internuclear distance” — the distance between the two protons at the moment the photon was absorbed — “made for radical differences in the ways the electrons were ejected.”
“When I saw the results of the molecular experiments, in which small changes in the internuclear distance produced large and unexpected changes in the electron ejection patterns, it immediately occurred to me that the differences were because of the molecule’s effects on electron correlations,” McCurdy says.
McCurdy had recently been working with Fernando Martín, a professor of chemistry at the Universidad Autónoma de Madrid, merging computational techniques developed by Martín with a method McCurdy, Rescigno, and others had developed for calculating systems of three charged particles. Martín and McCurdy extended these methods to the helium atom, a system that, technically speaking, has four charged particles. But because the helium atom’s two protons are bound together in the nucleus, the calculated distribution of electrons ejected by the absorption of an energetic photon tend to be quite symmetrical around the nucleus, with most pairs flying off in opposite directions.
The picture can look quite different for a hydrogen or deuterium molecule, in which a plot of the likelihood that electrons will be ejected at certain angles groups into lobes that grow increasingly asymmetric as the bond length between the two hydrogen atoms grows longer. McCurdy read this as the effect of the bond length on the correlation of the shared electrons. Indeed, this is what Weber and his colleagues speculated when they published the results of their deuterium photofragmentation studies in Nature in 2004.
Rescigno pointed out a fly in the ointment, however — namely that instead of being caused by electron correlations, large differences in ejection patterns caused by small differences in internuclear distance “could just be kinematics.”
In other words, the scattered electrons might be sharing some of the potential energy stored by the Coulomb repulsion between the two like-charged protons. The closer together these two nuclei are at the moment the photon breaks up the molecule, the more energy goes into the Coulomb explosion, some of which could be transmitted to the outgoing electrons and affect their flight paths.
How to decide between kinematic effects or electron correlations? The experimental results could not address the question, since all the data were collected at the same photon energy; whether the electrons were acquiring additional kinetic energy was unknown.
But, says Vanroose, “because we were doing computations, we could do experiments the experimenters couldn’t do. We had much more flexibility to fix the conditions.”
Using supercomputers at the Department of Energy’s National Energy Research Scientific Computing Center (NERSC) at Berkeley Lab, at UC Berkeley, and in Belgium, Vanroose was able to rerun the hydrogen molecule experiments “in silico,” this time with different photon energies, distributed so that the outgoing electrons always shared exactly the same kinetic energy no matter what the distance between the protons at the moment of photon absorption.
The results turned out to be remarkably similar in all cases. Even when kinetic energy made no contribution, the electrons flew off in patterns determined by the length of the bond between the nuclei. Therefore the differences were due almost entirely to the way the electrons were correlated in their orbital paths around the molecule’s two nuclei.
Martín of UA Madrid sees the new calculations, which are a complete numerical solution for the Schrödinger equation of the photoionization of H2, as “just the beginning. Probing the complicated physics of electron correlations will lead the way to more comprehensive methods combining theory and experiment to address some of the most pressing problems in chemistry.”
Vanroose credits their success to day-in, day-out collaboration between top-notch theorists and experimenters at Berkeley Lab, “who are talking to each other all the time. The ability of experimentalists to call on the latest computational techniques is good for both; it’s why we’re two years ahead of other theorists in this field.”
To Rescigno, the latest results show that “what began as blue-sky physics theory is now connecting with the nuts and bolts of practical experiment.”
Says McCurdy, “These large-scale theoretical calculations, stimulated by the need to interpret novel experiments at the ALS, are already stimulating new experiments and establishing a new line of inquiry at Berkeley Lab.”
“Complete photo-induced breakup of the H2 molecule as a probe of molecular electron correlation,” by Wim Vanroose, Fernando Martín, Thomas N. Rescigno, and C. William McCurdy, appears in the 16 December, 2005 issue of Science.
“Complete photo-fragmentation of the deuterium molecule,” by T. Weber, A. O. Czasch, O. Jagutzki, A. K. Müller, V. Mergel, A. Kheifets, E. Rotenberg, G. Meigs, M. H. Prior, S. Daveau, A. Landers, C. L. Cocke, T. Osipov, R. Díez Muiño, H. Schmidt-Böcking, and R. Dörner, appeared in the 23 September, 2004 issue of Nature.
Berkeley Lab is a U.S. Department of Energy national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California.
Media Contact
More Information:
http://www.lbl.govAll latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















