Solar fuels as generated by nature
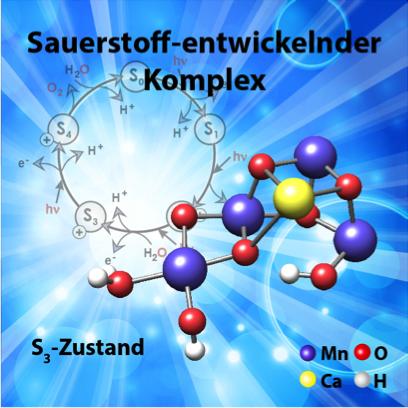
The structure of the manganese cluster as it is found in nature and prior to O-O bond formation. In the background, the water-splitting cycle with the intermediate states S0 to S4. © Diagram: MPI for Chemical Energy Conversion
Society’s energy supply problems could be solved in the future using a model adopted from nature. During photosynthesis, plants, algae and some species of bacteria produce sugars and other energy-rich substances (i.e. fuels) using solar energy. A team headed by researchers from the Max Planck Institute for Chemical Energy Conversion in Mülheim an der Ruhr is currently developing experimental methods to ascertain how this process occur in nature.
The scientists are investigating a particularly important cofactor involved in photosysthesis, a manganese-calcium complex, which uses solar energy to split water into molecular oxygen. They have determined the exact structure of this complex at a crucial stage in this chemical reaction. This has led to a detailed suggestion as to how molecular oxygen, O2, is formed at this metal complex. Through these new insights into photosynthesis, the scientists have provided a blueprint for synthetic systems that could store sunlight energy in chemical energy carriers.
For over three billion years, nature has been using sunlight as its primary energy source in photosynthesis. In the course of this process, plants, algae and cyanobacteria (blue-green algae) use sunlight to split water and produce energy-rich chemical compounds from carbon dioxide (CO2). The end product is carbohydrates which, in nature, act as solar fuels in the living cell.
Although the basic reactions involved in photosynthesis has been known for a long time, researchers from the Max Planck Institute for Chemical Energy Conversion in Mülheim an der Ruhr and the Commissariat à l'Énergie Atomique (CEA) in Saclay, France, have now succeeded in explaining important details of the light-induced water splitting process. As a result, they have refined the scientific basis for the generation of environmentally-friendly, low-cost solar fuels through artificial photosynthesis using sunlight and water, a development that could enable society to end its dependency on fossil fuels such as oil, coal and natural gas.
Light-induced catalytic water splitting takes place at a metal complex which is embedded in a large membrane protein (photosystem II). This complex is composed of four manganese atoms (Mn) and one calcium atom (Ca), which are held together through a network of oxygen bridges (see image). This water-oxidising or oxygen-evolving complex undergoes a complicated cycle that releases electrons and protons, hence ultimately hydrogen, and molecular oxygen.
In an article published this week in the journal Science, the German-French research team presents the structure of this manganese-calcium complex directly before the production of oxygen. This insight into a key stage of plant photosynthesis is highly significant: it provides a more detailed understanding of the mechanism involved in photosynthesis and will enable the development of synthetic systems for light-induced water splitting based on this model.
The study is the result of a close cooperation between the Departments of Biophysical Chemistry and Molecular Theory at the Max Planck Institute for Chemical Energy Conversion under the leaderships of Wolfgang Lubitz and Frank Neese. Within these departments, Nicholas Cox and Dimitrios Pantazis assembled an interdisciplinary team that aims to gain a better understanding of the molecular details of water-splitting in nature.
The first challenge faced by the researchers involved the extraction and purification of photosystem II with a fully intact water-splitting complex from the original organism, a thermophilic cyanobacterium, which is found in hot springs and volcanoes in Japan and is very robust. To fulfil the very stringent requirements regarding the quality of the preparation, the researchers in Saclay had to carry out several years of development work in cooperation with researchers from Japan.
The second challenge the research team encountered concerned the characterisation of the manganese complex in photosystem II during the different stages of water-splitting. The researchers from the Biophysical Chemistry Department of the Mülheim-based Max Planck Institute overcame this hurdle with the help of electron paramagnetic resonance (EPR). This technique makes it possible to visualise the distribution of the electrons in a molecule or metal complex and thus provides deep insight into the individual stages of water-splitting. “These measurements generated new information and enabled the solvation of problems concerning the detailed analysis of molecular structures in the reaction cycle that are not accessible using other methods,” says Dr Alain Boussac from the CEA Saclay.
Finally, the third challenge consisted in using the information obtained to produce a complete structural model of the biocatalyst. The calculations necessary for this process were facilitated using new theoretical methods and the supercomputers at the Department of Molecular Theory at the Max Planck Institute. In this way, the researchers succeeded in showing that during the late phase of the reaction cycle, a second water molecule binds next to an active oxygen atom in the complex and releases a proton. This leads to the formation of the O-O bond in the next step.
Thanks to this decoding of the structure and function of the water-splitting catalyst in photosystem II at atomic level, an explanation of the water splitting mechanism is now within reach. This knowledge enables the identification of important criteria for the design of similar synthetic catalysts that split water using environmentally-friendly, low-cost and easily available elements. At present, expensive platinum and other rare metals or metal complexes are widely used for this purpose. This makes the large-scale production of renewable energy carriers (fuels) like hydrogen very expensive, or even impossible.
With the help of bio-inspired catalysts, hydrogen or another solar fuel could be produced cheaply through the combination of solar power devices with water-splitting catalysts for the generation of solar fuels instead of electricity. This would enable the energy sector to overcome the main problems associated with solar power: sunlight is not available around the clock as an energy source, and electricity is not very wellsuited for running motor vehicles. In contrast, the solar fuel concept enables the direct storage of solar energy in chemical compounds and, therefore, the use of this energy at any time or place.
“Synthetic solar fuels open up wide-ranging possibilities for renewable energy technologies, in particular for the transport and infrastructure sectors, which are still reliant on fossil fuels,” says Professor Wolfgang Lubitz, Director at the Max Planck Institute for Chemical Energy Conversion. “An efficient light-driven, water splitting catalyst based on common metals such as manganese would represent huge progress here. The insight gained into nature’s water splitting enzyme through this research has laid the foundations for such developments.”
Contact
Phone: +49 208 306-3614
Fax: +49 208 306-3955
Media Contact
More Information:
http://www.mpg.de/8373743/photosynthetic-water-splittingAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Simplified diagnosis of rare eye diseases
Uveitis experts provide an overview of an underestimated imaging technique. Uveitis is a rare inflammatory eye disease. Posterior and panuveitis in particular are associated with a poor prognosis and a…

Targeted use of enfortumab vedotin for the treatment of advanced urothelial carcinoma
New study identifies NECTIN4 amplification as a promising biomarker – Under the leadership of PD Dr. Niklas Klümper, Assistant Physician at the Department of Urology at the University Hospital Bonn…
A novel universal light-based technique
…to control valley polarization in bulk materials. An international team of researchers reports in Nature a new method that achieves valley polarization in centrosymmetric bulk materials in a non-material-specific way…





















