Scientists Find Dissimilar Proteins Evolved Similar 7-Part Shape
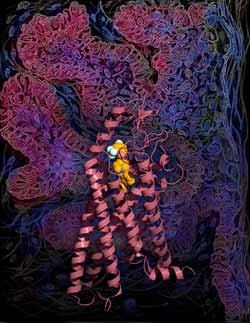
Image courtesy of the Stevens lab, The Scripps Research Institute.<br> <br>The new research shows the smoothened receptor (SMO) has remarkable structural similarities to genetically unrelated proteins.<br>
Described this week in the journal Nature, the work brings attention to what scientists have thought of as a family of molecules called the G protein-coupled receptors (GPCRs). Many GPCRs are important targets for drug design. However, the new work suggests that GPCRs may, in fact, be a subset of a larger group.
“This work highlights the need to modify how we classify the GPCR family,” said TSRI Professor Raymond Stevens, PhD, the senior author on the study. “The study suggests we should start calling the family 7-transmembrane receptors, which has been proposed by others before, to better reflect the diversity of the family, both structurally and in terms of function.”
The new classification would include proteins with similar shapes to GPCRs—like the smoothened receptor (SMO), which was the subject of the new research.
Different Genes, Same Structure
In the study, the TSRI team solved the high-resolution structure of SMO, which is the first non-class A GPCR structure published to date (class A GPCRs are also known as rhodopsin-like GPCRs). The results showed the molecule is nearly identical to the classic GPCR shape, even though it bears almost no similarity in terms of genetic sequence.
Often, two proteins with very different sequences have different structures, said Chong Wang, a graduate student at TSRI’s Kellogg School of Science and Technology who is the first author on the study.
“These receptors are very different—less than 10 percent sequence identity, and yet they have the same 7-transmembrane helical fold,” Wang added.
“This is a great example of structural conservation of the 7-transmembrane fold,” said Stevens. “A key question is, why the magic number 7?”
Potential Target for Drug Design
The work is also significant because the SMO protein itself is a potential target for drug design.
SMO is important for proper growth in the early stages of mammalian development, and animals with deficiencies in the activities of this protein develop severe deformities in the womb. The initial discovery was made in 1957, when sheep in Idaho ate corn lily containing cyclopamine, and newborns were observed to develop a single eye—a characteristic for which the condition, known as “cyclopia,” is named. In work published in the journal Nature in 2000, Stanford University researchers Philip Beachy and Matthew Scott found cyclopamine inhibits the SMO receptor.
The body reduces its need for SMO in adulthood, and its activity is usually curtailed. However, later in life the protein can also play a role in disease, this time by helping cancerous tumors grow. SMO receptor inhibition has been harnessed as a means to reduce basal cell carcinoma, a common form of skin cancer.
The discovery of the structure of SMO may help researchers develop new molecules to treat cancer and other diseases.
“The structure of the human smoothened receptor bound to an anti-cancer compound will help us understand the receptor’s role in cancer, as well as its role in the normal process of embryonic development,” said Jean Chin, PhD, of the National Institutes of Health’s National Institute of General Medical Sciences, which partly supported the research. “In addition, comparison of smoothened’s unique structure with those of the more conventional GPCRs will teach us a lot about how these receptors respond to the many therapeutics they interact with.”
The Nature article, “Structure of the human smoothened receptor bound to an antitumor agent,” was authored by Chong Wang, Huixian Wu, Vsevolod Katritch, Gye Won Han, Xi-Ping Huang, Wei Liu, Fai Yiu Siu, Bryan L. Roth, Vadim Cherezov and Raymond C. Stevens. For more information, see: http://dx.doi.org/10.1038/nature12167
This work was funded by the National Institutes of Health through grant numbers P50 GM073197, U54 GM094618, F32 DK088392, R01 MH61887, U19 MH82441 and R01 DA27170. Additional support was provided through the National Institute of Mental Health Psychoactive Drug Screening Program and the Michael Hooker Chair of Pharmacology to Bryan Roth at the University of North Carolina.
About The Scripps Research Institute
The Scripps Research Institute (TSRI) is one of the world's largest independent, not-for-profit organizations focusing on research in the biomedical sciences. TSRI is internationally recognized for its contributions to science and health, including its role in laying the foundation for new treatments for cancer, rheumatoid arthritis, hemophilia, and other diseases. An institution that evolved from the Scripps Metabolic Clinic founded by philanthropist Ellen Browning Scripps in 1924, the institute now employs about 3,000 people on its campuses in La Jolla, CA, and Jupiter, FL, where its renowned scientists—including three Nobel laureates—work toward their next discoveries. The institute's graduate program, which awards PhD degrees in biology and chemistry, ranks among the top ten of its kind in the nation. For more information, see www.scripps.edu.
Media Contact
More Information:
http://www.scripps.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…





















